|
Dry Cell
upLine art drawing of a dry cell: 1. brass cap, 2. plastic seal, 3. expansion space, 4. porous cardboard, 5. zinc can, 6. carbon rod, 7. chemical mixture A dry cell is a type of electric battery, commonly used for portable electrical devices. Unlike wet cell batteries, which have a liquid electrolyte, dry cells use an electrolyte in the form of a paste, and are thus less susceptible to leakage. The dry cell was developed in 1886 by the German scientist Carl Gassner, after development of wet zinc–carbon batteries by Georges Leclanché in 1866. A type of dry cell was also developed by the Japanese Sakizō Yai in 1887. History left, upDry cell battery by Wilhelm Hellesen 1890 Many experimenters tried to immobilize the electrolyte of an electrochemical cell to make it more convenient to use. The Zamboni pile of 1812 is a high-voltage dry battery but capable of delivering only minute currents. Various experiments were made with cellulose, sawdust, spun glass, asbestos fiber ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dry Cell (PSF)
upLine art drawing of a dry cell: 1. brass cap, 2. plastic seal, 3. expansion space, 4. porous cardboard, 5. zinc can, 6. carbon rod, 7. chemical mixture A dry cell is a type of electric battery, commonly used for portable electrical devices. Unlike wet cell batteries, which have a liquid electrolyte, dry cells use an electrolyte in the form of a paste, and are thus less susceptible to leakage. The dry cell was developed in 1886 by the German scientist Carl Gassner, after development of wet zinc–carbon batteries by Georges Leclanché in 1866. A type of dry cell was also developed by the Japanese Sakizō Yai in 1887. History left, upDry cell battery by Wilhelm Hellesen 1890 Many experimenters tried to immobilize the electrolyte of an electrochemical cell to make it more convenient to use. The Zamboni pile of 1812 is a high-voltage dry battery but capable of delivering only minute currents. Various experiments were made with cellulose, sawdust, spun glass, asbestos fibers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ray-O-Vac Ad 1949
Spectrum Brands Holdings, Inc. is an American diversified company. Headquartered in Middleton, Wisconsin, it was established in 2005 as the successor company to Rayovac Corporation. It is one of the Fortune 500 companies, and among the largest of its kind on the list. The company manufactures and markets home appliances under the Remington, Black & Decker, George Foreman, and Russell Hobbs brand names, lawn and garden care products under the Spectracide and Garden Safe brand names, and insect repellents under the Cutter and Repel brand names. Spectrum owns several pet care companies, both in the aquarium supply and companion animal trades. In the aquarium business, Spectrum owns Tetra, Whisper, Marineland, Perfecto, Jungle, Instant Ocean, Visi-Therm, and other product lines. Companion animal lines consist of Dingo, Nature's Miracle, Lazy Pet, Wonderbox, Furminator, IAMS, Eukanuba and others. Both aquarium lines and companion lines are concentrated into Spectrum's United Pet Group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in which positive charges move. Electrons have a negative electrical charge, so the movement of electrons is opposite to that of the conventional current flow. Consequently, the mnemonic ''cathode current departs'' also means that electrons flow ''into'' the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode. The electrode through which conventional current flows the other way, into the device, is termed an anode. Charge flow Conventional current flows from cathode to anode outside of the cell or device (with electrons moving in the opposite direction), regardless of the cell or device type and operating mode. Cathode polarity with respect to the anode can be positive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an ...—its atom making four electrons available to form covalent bond, covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, Carbon-12, C and Carbon-13, C being stable, while Carbon-14, C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the Timeline of chemical element discoveries#Ancient discoveries, few elements known since antiquity. Carbon is the 15th Abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the Abundance of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ACID, for "anode current into device". The direction of conventional current (the flow of positive charges) in a circuit is opposite to the direction of electron flow, so (negatively charged) electrons flow out the anode of a galvanic cell, into an outside or external circuit connected to the cell. For example, the end of a household battery marked with a "-" (minus) is the anode. In both a galvanic cell and an electrolytic cell, the anode is the electrode at which the oxidation reaction occurs. In a galvanic cell the anode is the wire or plate having excess negative charge as a result of the oxidation reaction. In an electrolytic cell, the anode is the wire or plate upon which excess positive charge is imposed. As a result of this, anion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size.The elements are from different metal groups. See periodic table. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Battery
An alkaline battery (IEC code: L) is a type of primary battery where the electrolyte (most commonly potassium hydroxide) has a pH value above 7. Typically these batteries derive energy from the reaction between zinc metal and manganese dioxide, nickel and cadmium, or nickel and hydrogen. Compared with zinc–carbon batteries of the Leclanché cell or zinc chloride types, alkaline batteries have a higher energy density and longer shelf life, yet provide the same voltage. The alkaline battery gets its name because it has an alkaline electrolyte of potassium hydroxide (KOH) instead of the acidic ammonium chloride (NH4Cl) or zinc chloride (ZnCl2) electrolyte of the zinc–carbon batteries. Other battery systems also use alkaline electrolytes, but they use different active materials for the electrodes. Alkaline batteries account for 80% of manufactured batteries in the US and over 10 billion individual units produced worldwide. In Japan, alkaline batteries account for 46% of all ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volt
The volt (symbol: V) is the unit of electric potential, electric potential difference (voltage), and electromotive force in the International System of Units (SI). It is named after the Italian physicist Alessandro Volta (1745–1827). Definition One volt is defined as the electric potential between two points of a conducting wire when an electric current of one ampere dissipates one watt of power between those points. Equivalently, it is the potential difference between two points that will impart one joule of energy per coulomb of charge that passes through it. It can be expressed in terms of SI base units ( m, kg, second, s, and ampere, A) as : \text = \frac = \frac = \frac. It can also be expressed as amperes times ohms (current times resistance, Ohm's law), webers per second (magnetic flux per time), watts per ampere (power per current), or joules per coulomb (energy per charge), which is also equivalent to electronvolts per elementary charge: : \text = \tex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
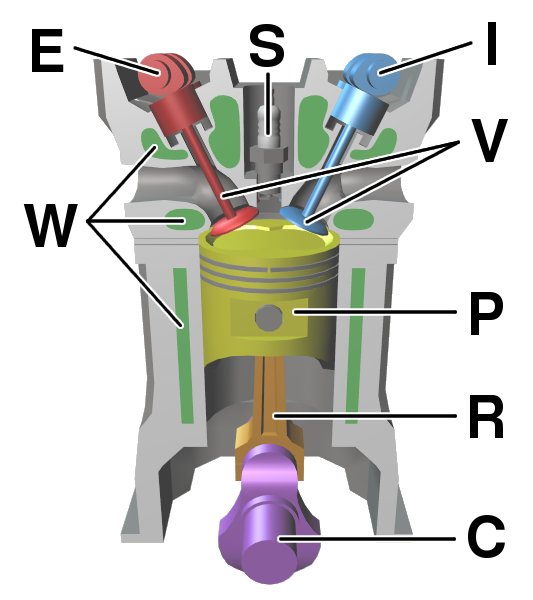
Internal Combustion Engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combustion engine, the expansion of the high-temperature and high-pressure gases produced by combustion applies direct force to some component of the engine. The force is typically applied to pistons ( piston engine), turbine blades (gas turbine), a rotor (Wankel engine), or a nozzle ( jet engine). This force moves the component over a distance, transforming chemical energy into kinetic energy which is used to propel, move or power whatever the engine is attached to. This replaced the external combustion engine for applications where the weight or size of an engine was more important. The first commercially successful internal combustion engine was created by Étienne Lenoir around 1860, and the first modern internal combustion engine, known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gel Battery
A valve regulated lead–acid (VRLA) battery, commonly known as a sealed lead–acid (SLA) battery, is a type of lead–acid battery characterized by a limited amount of electrolyte ("starved" electrolyte) absorbed in a plate separator or formed into a gel; proportioning of the negative and positive plates so that oxygen recombination is facilitated within the cell; and the presence of a relief valve that retains the battery contents independent of the position of the cells. There are two primary types of VRLA batteries, absorbent glass mat (AGM) and gel cell (gel battery). The lead–acid gel batteries contain a mixture of sulfuric acid and finely divided silica. This mixture forms a thick paste or gel, thereby giving the batteries the name - Gel Cell. Gel batteries can be made with either flat or tubular positive plates. AGM batteries feature fiberglass mesh or an ultra thin glass mat (called AGM separator) between the battery plates which serves to contain the electrolyte and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spillage
In industrial production, spillage is the loss of production output due to production of a series of defective or unacceptable products which must be rejected. Spillage is an often costly event which occurs in manufacturing when a process degradation or failure occurs that is not immediately detected and corrected, and in which defective or reject product therefore continues to be produced for some extended period of time. Spillage results in costs due to lost production volume, excessive scrap, delayed delivery of product, and wastage of human and capital equipment resources. Minimization of the occurrence and duration of manufacturing spillage requires that closed-loop control and associated process monitoring and metrology functions be integrated into critical steps of the overall manufacturing process. The extent to which process control An industrial process control in continuous production processes is a discipline that uses industrial control systems to achieve a pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |