|
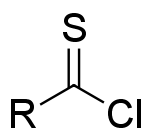
Dithiocarboxylic Acid
Dithiocarboxylic acids are organosulfur compounds with the formula . They are the dithia analogues of carboxylic acids. A closely related and better studied family of compounds are the monothiocarboxylic acids, with the formula . Dithiocarboxylic acids are about 3x more acidic than the monothiocarboxylic acids. Thus, for dithiobenzoic acid pKa = 1.92. Such compounds are commonly prepared by the reaction of carbon disulfide with a Grignard reagent: : : This reaction is comparable to the formation of carboxylic acids using a Grignard reagent and carbon dioxide. Dithiocarboxylate salts readily S-alkylate to give dithiocarboxylate esters: :{{chem2, RCS2Na + R'Cl -> RCS2R' + NaCl Aryldithiocarboxylic acids, e.g., dithiobenzoic acid, chlorinate to give the thioacyl chloride In organic chemistry, thioacyl chloride is a functional group of the type RC(S)Cl, where R is an organic substituent. Thioacyl chlorides are analogous to acid chlorides, but much rarer and less robust. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur Compound
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two ( cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tellurium compounds. A classical chemical test for the detection of sulfur co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiocarboxylic Acid
In organic chemistry, thiocarboxylic acids are organosulfur compounds related to carboxylic acids by replacement of one of the oxygen atoms with a sulfur atom. Two tautomers are possible: a Thioketone, thione form () and a thiol form (). These are sometimes also referred to as "carbothioic ''O''-acid" and "carbothioic ''S''-acid" respectively. Of these the thiol form is most common (e.g. thioacetic acid). A naturally occurring thiocarboxylic acid is pyridine-2,6-dicarbothioic acid, a siderophore. Synthesis Thiocarboxylic acids are typically prepared by salt metathesis from the acid chloride, as in the following conversion of benzoyl chloride to thiobenzoic acid using potassium hydrosulfide according to the following idealized equation: :C6H5C(O)Cl + KSH -> C6H5C(O)SH + KCl Reactions At neutral pH, thiocarboxylic acids are fully ionized. Thiocarboxylic acids are about 100 times more acidic than the analogous carboxylic acids. For PhC(O)SH pKa = 2.48 vs 4.20 for benzoic acid, PhC( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent. It has an "ether-like" odor, but commercial samples are typically contaminated with foul-smelling impurities.. It is of comparable toxicity to carbon monoxide. History In 1796, the German chemist Wilhelm August Lampadius (1772–1842) first prepared carbon disulfide by heating pyrite with moist charcoal. He called it "liquid sulfur" (''flüssig Schwefel''). The composition of carbon disulfide was finally determined in 1813 by the team of the Swedish chemist Jöns Jacob Berzelius (1779–1848) and the Swiss-British chemist Alexander Marcet (1770–1822). Their analysis was consistent with an empirical formula of CS2. Occurrence, manufacture, properties Small amounts of carbon disulfide are released by volcanic eruptio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. In this aspect, they are similar to organolithium reagents. Pure Grignard reagents are extremely reactive solids. They are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran; which are relatively stable as long as water is excluded. In such a medium, a Grignard reagent is invariably present as a complex with the magnesium atom conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
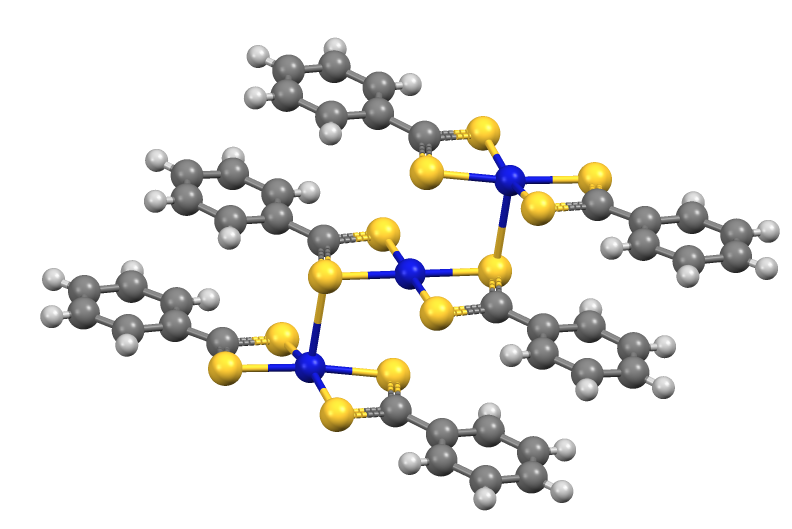
Dithiobenzoic Acid
Dithiobenzoic acid is the organosulfur compound with the formula C6H5CS2H. It is a dithiocarboxylic acid, an analogue of benzoic acid, but more acidic and deeply colored. Synthesis and reactions It can be prepared by sulfiding benzal chloride: :C6H5CCl3 + 4 KSH → C6H5CS2K + 3 KCl + 2 H2S :C6H5CS2K + H+ → C6H5CS2H + K+ It also arises by the reaction of the Grignard reagent phenylmagnesium bromide with carbon disulfide, followed by acidification: :C6H5MgBr + CS2 → C6H5CS2MgBr :C6H5CS2MgBr + HCl → C6H5CS2H + MgBrCl It is about 100x more acidic than benzoic acid Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, .... Its conjugate base, dithiobenzoate, undergoes S-alkylation to give dithiocarboxylate esters. Similarly, dithiobenzoate reacts with "soft" metal salts to give c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioacyl Chloride
In organic chemistry, thioacyl chloride is a functional group of the type RC(S)Cl, where R is an organic substituent. Thioacyl chlorides are analogous to acid chlorides, but much rarer and less robust. The best studied is thiobenzoyl chloride, a purple oil first prepared by chlorination of dithiobenzoic acid with a combination of chlorine and thionyl chloride. A more modern preparation employs phosgene as the chlorinating agent,{{cite journal, doi= 10.1002/zfch.19750150904, authors=Viola, H.;Mayer, R., journal=Z. Chem., title=Eine neue Darstellungsmethode für aromatische Thiocarbonsäurechloride, trans-title=A New Preparation Route for Aromatic Thiocarboxylic Acid Chlorides, year=1975, volume=15, issue=9, page=348 this also generates carbonyl sulfide as a by-product. PhCS2H + COCl2 → PhC(S)Cl + HCl + COS The most common thioacyl chloride is thiophosgene Thiophosgene is a red liquid with the formula . It is a molecule with trigonal planar geometry. There are two reactive C� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Acids
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are relatively stronger acids. Alcohols, with –OH, can act as acids but they are usually very weak. The relative stability of the conjugate base of the acid determines its acidity. Other groups can lsoconfer acidity, usually weakly: the thiol group –SH, the enol group, and the phenol group. In biological systems, organic compounds containing these groups are generally referred to as organic acids. A few common examples include: * lactic acid * acetic acid * formic acid * citric acid * oxalic acid * uric acid * malic acid * tartaric acid Characteristics In general, organic acids are weak acids and do not dissociate completely in water, whereas the strong mineral acids do. Lower molecular mass organic acids such as formic and lactic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |