Thioacyl Chloride on:
[Wikipedia]
[Google]
[Amazon]
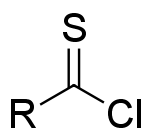 In
In
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
, thioacyl chloride is a functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
of the type RC(S)Cl, where R is an organic substituent. Thioacyl chlorides are analogous to acid chlorides, but much rarer and less robust. The best studied is thiobenzoyl chloride, a purple oil first prepared by chlorination of dithiobenzoic acid with a combination of chlorine and thionyl chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately per year bein ...
.
A more modern preparation employs phosgene as the chlorinating agent,{{cite journal, doi= 10.1002/zfch.19750150904, authors=Viola, H.;Mayer, R., journal=Z. Chem., title=Eine neue Darstellungsmethode für aromatische Thiocarbonsäurechloride, trans-title=A New Preparation Route for Aromatic Thiocarboxylic Acid Chlorides, year=1975, volume=15, issue=9, page=348 this also generates carbonyl sulfide
Carbonyl sulfide is the chemical compound with the linear formula OCS. It is a colorless flammable gas with an unpleasant odor. It is a linear molecule consisting of a carbonyl group double bonded to a sulfur atom. Carbonyl sulfide can be consi ...
as a by-product.
PhCS2H + COCl2 → PhC(S)Cl + HCl + COS
The most common thioacyl chloride is thiophosgene
Thiophosgene is a red liquid with the formula . It is a molecule with trigonal planar geometry. There are two reactive C–Cl bonds that allow it to be used in diverse organic syntheses.
Preparation
is prepared in a two-step process from carbon ...
.
References