|
Dimethyl Sulphide
Dimethyl sulfide (DMS) or methylthiomethane is an organosulfur compound with the formula (CH3)2S. Dimethyl sulfide is a flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize, cabbage, beetroot, and seafoods. It is also an indication of bacterial contamination in malt production and brewing. It is a breakdown product of dimethylsulfoniopropionate (DMSP), and is also produced by the bacterial metabolism of methanethiol. Occurrence and production DMS originates primarily from DMSP, a major secondary metabolite in some marine algae. DMS is the most abundant biological sulfur compound emitted to the atmosphere. Emission occurs over the oceans by phytoplankton. DMS is also produced naturally by bacterial transformation of dimethyl sulfoxide (DMSO) waste that is disposed of into sewers, where it can cause environmental odor problems. DMS is oxidized in the marine atmosp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space-filling Model
In chemistry, a space-filling model, also known as a ''calotte model'', is a type of three-dimensional (3D) molecular model where the atoms are represented by spheres whose radii are proportional to the radii of the atoms and whose center-to-center distances are proportional to the distances between the atomic nuclei, all in the same scale. Atoms of different chemical elements are usually represented by spheres of different colors. Space-filling calotte models are also referred to as CPK models after the chemists Robert Corey, Linus Pauling, and Walter Koltun, who over a span of time developed the modeling concept into a useful form. They are distinguished from other 3D representations, such as the ball-and-stick and skeletal models, by the use of the "full size" space-filling spheres for the atoms. The models are tactile and manually rotatable. They are useful for visualizing the effective shape and relative dimensions of a molecule, and (because of the rotatability) the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brewing
Brewing is the production of beer by steeping a starch source (commonly cereal grains, the most popular of which is barley) in water and #Fermenting, fermenting the resulting sweet liquid with Yeast#Beer, yeast. It may be done in a brewery by a commercial brewer, at home by a homebrewer, or communally. Brewing has taken place since around the 6th millennium BC, and archaeological evidence suggests that emerging civilizations, including ancient Egypt and Mesopotamia, brewed beer. Since the nineteenth century the #brewing industry, brewing industry has been part of most western economies. The basic ingredients of beer are water and a Fermentation, fermentable starch source such as malted barley. Most beer is fermented with a brewer's yeast and flavoured with hops. Less widely used starch sources include millet, sorghum and cassava. Secondary sources (adjuncts), such as maize (corn), rice, or sugar, may also be used, sometimes to reduce cost, or to add a feature, such as addin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cloud Condensation Nuclei
Cloud condensation nuclei (CCNs), also known as cloud seeds, are small particles typically 0.2 µm, or one hundredth the size of a cloud droplet. CCNs are a unique subset of aerosols in the atmosphere on which water vapour condenses. This can affect the radiative properties of clouds and the overall atmosphere. Water requires a non-gaseous surface to make the transition from a vapour to a liquid; this process is called condensation. In the atmosphere of Earth, this surface presents itself as tiny solid or liquid particles called CCNs. When no CCNs are present, water vapour can be supercooled at about for 5–6 hours before droplets spontaneously form. This is the basis of the cloud chamber for detecting subatomic particles. The concept of CCN is used in cloud seeding, which tries to encourage rainfall by seeding the air with condensation nuclei. It has further been suggested that creating such nuclei could be used for marine cloud brightening, a climate engineering techni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aerosols
An aerosol is a suspension of fine solid particles or liquid droplets in air or another gas. Aerosols can be natural or anthropogenic. Examples of natural aerosols are fog or mist, dust, forest exudates, and geyser steam. Examples of anthropogenic aerosols include particulate air pollutants, mist from the discharge at hydroelectric dams, irrigation mist, perfume from atomizers, smoke, steam from a kettle, sprayed pesticides, and medical treatments for respiratory illnesses. When a person inhales the contents of a vape pen or e-cigarette, they are inhaling an anthropogenic aerosol. The liquid or solid particles in an aerosol have diameters typically less than 1 μm (larger particles with a significant settling speed make the mixture a suspension, but the distinction is not clear-cut). In general conversation, ''aerosol'' often refers to a dispensing system that delivers a consumer product from a can. Diseases can spread by means of small droplets in the breat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released may boi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanesulfonic Acid
Methanesulfonic acid (MsOH) or methanesulphonic acid (in British English) is an organosulfuric, colorless liquid with the chemical formula and structure . It is the simplest of the alkylsulfonic acids (). Salts and esters of methanesulfonic acid are known as mesylates (or methanesulfonates, as in ethyl methanesulfonate). It is hygroscopic in its concentrated form. Methanesulfonic acid can dissolve a wide range of metal salts, many of them in significantly higher concentrations than in hydrochloric acid (HCl) or sulfuric acid (). Applications Methanesulfonic acid is used as an acid catalyst in organic reactions because it is a non-volatile, strong acid that is soluble in organic solvents. It is convenient for industrial applications because it is liquid at ambient temperature, while the closely related ''p''-toluenesulfonic acid (PTSA) is solid. However, in a laboratory setting, solid PTSA is more convenient. Methanesulfonic acid can be used in the generation of borane (BH3) b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylsulfonylmethane
Methylsulfonylmethane (MSM) is an organosulfur compound with the formula (CH3)2SO2. It is also known by several other names including methyl sulfone and dimethyl sulfone (DMSO2). This colorless solid features the sulfonyl functional group and is the simplest of the sulfones. It is considered relatively inert chemically and is able to resist decomposition at elevated temperatures. It occurs naturally in some primitive plants, is present in small amounts in many foods and beverages, and is marketed as a dietary supplement. It is sometimes used as a cutting agent for illicitly manufactured methamphetamine. It is also commonly found in the atmosphere above marine areas, where it is used as a carbon source by the airborne bacteria ''Afipia''. Oxidation of dimethyl sulfoxide produces the sulfone, both under laboratory conditions and metabolically. Use as a solvent Because of its polarity and thermal stability, MSM has been used industrially as a high-temperature solvent. For example, di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of copper extraction and the burning of sulfur- bearing fossil fuels. Structure and bonding SO2 is a bent molecule with ''C''2v symmetry point group. A valence bond theory approach considering just ''s'' and ''p'' orbitals would describe the bonding in terms of resonance between two resonance structures. The sulfur–oxygen bond has a bond order of 1.5. There is support for this simple approach that does not invoke ''d'' orbital participation. In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of +1. Occurrence Sulfur dioxide is found on Earth and exists in very small concentrations and in the atmosphere at about 1 ppm. On other planets, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO has the unusual property that many individuals perceive a garlic-like taste in the mouth after DMSO makes contact with their skin. In terms of chemical structure, the molecule has idealized Cs symmetry. It has a trigonal pyramidal molecular geometry consistent with other three-coordinate S(IV) compounds, with a nonbonded electron pair on the approximately tetrahedral sulfur atom. Synthesis and production Dimethyl sulfoxide was first synthesized in 1866 by the Russian scientist Alexander Zaytsev, who reported his findings in 1867. Dimethyl sulfoxide is produced industrially from dimethyl sulfide, a by-product of the Kraf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
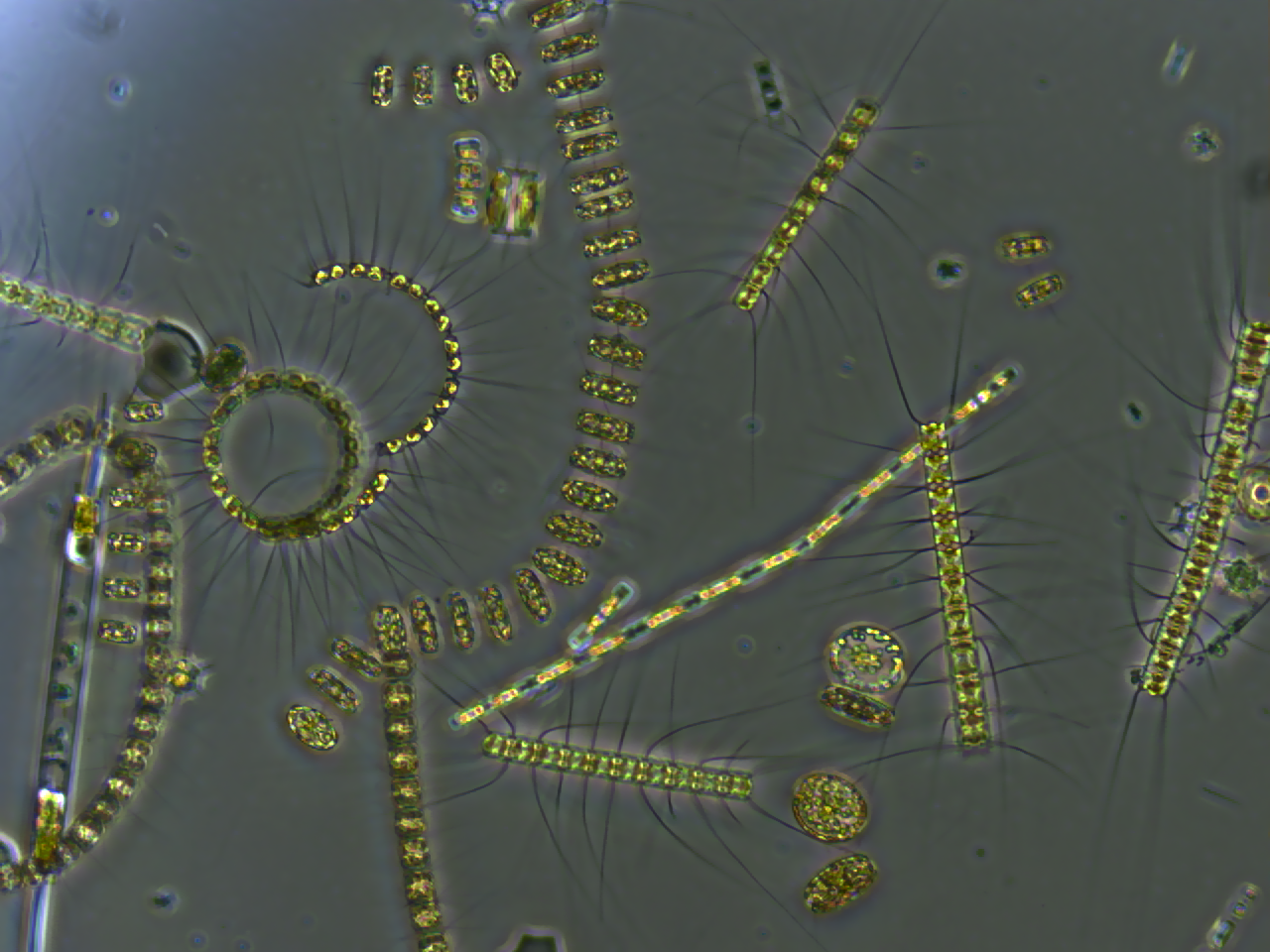
Phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater ecosystems. The name comes from the Greek words (), meaning 'plant', and (), meaning 'wanderer' or 'drifter'. Phytoplankton obtain their energy through photosynthesis, as do trees and other plants on land. This means phytoplankton must have light from the sun, so they live in the well-lit surface layers (euphotic zone) of oceans and lakes. In comparison with terrestrial plants, phytoplankton are distributed over a larger surface area, are exposed to less seasonal variation and have markedly faster turnover rates than trees (days versus decades). As a result, phytoplankton respond rapidly on a global scale to climate variations. Phytoplankton form the base of marine and freshwater food webs and are key players in the global carbon cycle. They account for about half of global photosynthetic activity and at least half of the oxygen production, despite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ocean
The ocean (also the sea or the world ocean) is the body of salt water that covers approximately 70.8% of the surface of Earth and contains 97% of Earth's water. An ocean can also refer to any of the large bodies of water into which the world ocean is conventionally divided."Ocean." ''Merriam-Webster.com Dictionary'', Merriam-Webster, [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Earth's Atmosphere
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing for liquid water to exist on the Earth's surface, absorbing ultraviolet solar radiation, warming the surface through heat retention (greenhouse effect), and reducing temperature extremes between day and night (the diurnal temperature variation). By mole fraction (i.e., by number of molecules), dry air contains 78.08% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon dioxide, and small amounts of other gases. Air also contains a variable amount of water vapor, on average around 1% at sea level, and 0.4% over the entire atmosphere. Air composition, temperature, and atmospheric pressure vary with altitude. Within the atmosphere, air suitable for use in photosynthesis by terrestrial plants and breathing of terrestrial animals is found only in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |