|
Dimemebfe
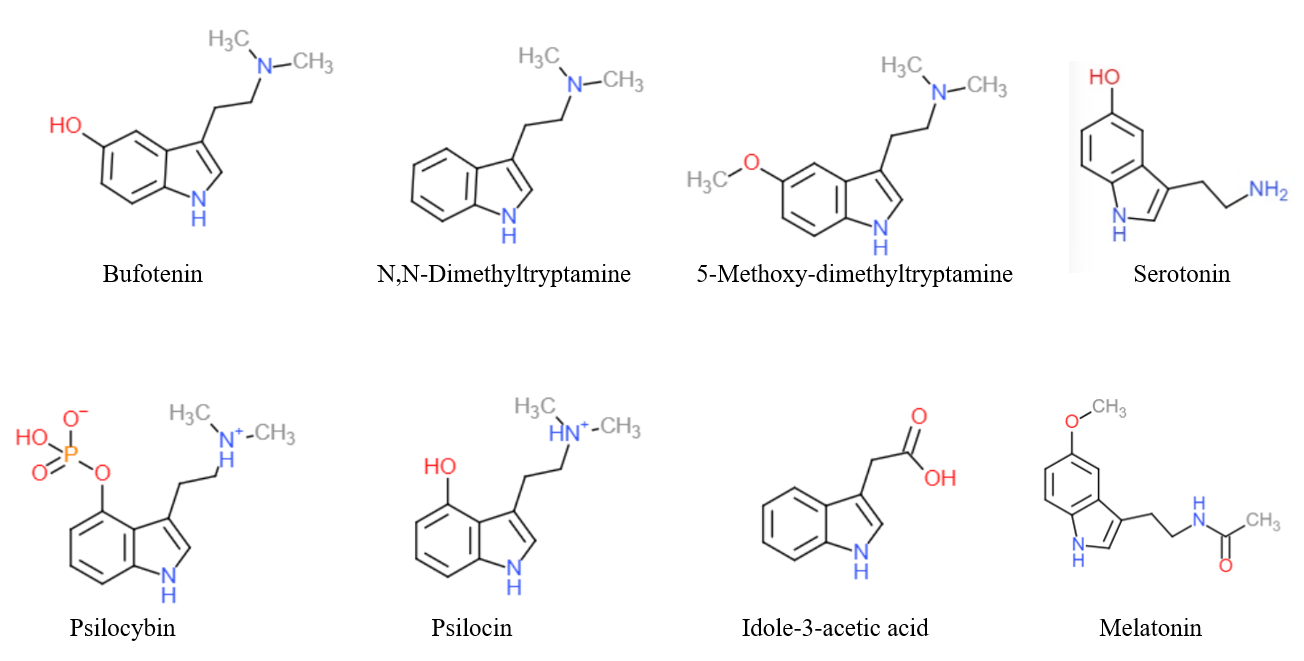
Dimemebfe (5-MeO-BFE) is a recreational drug and research chemical. It acts as an agonist for the 5-HT1A and 5-HT2 family of serotonin receptors. It is related in structure to the psychedelic tryptamine derivative 5-MeO-DMT, but with the indole nitrogen replaced by oxygen, making dimemebfe a benzofuran derivative. It is several times less potent as a serotonin agonist than 5-MeO-DMT and with relatively more activity at 5-HT1A, but still shows strongest effects at the 5-HT2 family of receptors. Legal status Dimemebfe is a Schedule I controlled substance in the US state of Alabama. See also * 5-MeO-DiBF 5-MeO-DiBF is a psychedelic that has been sold online as a designer drug and was first definitively identified in December 2015 by a forensic laboratory in Slovenia. It is thought to act as an agonist for the 5-HT1A and 5-HT2 family of serot ... References Benzofuranethanamines Designer drugs Serotonin receptor agonists Benzofuran ethers at the benzene ring ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-MeO-DMT
5-MeO-DMT (5-methoxy-''N'',''N''-dimethyltryptamine) or O-methyl-bufotenin is a psychedelic of the tryptamine class. It is found in a wide variety of plant species, and also is secreted by the glands of at least one toad species, the Colorado River toad. Like its close relatives DMT and bufotenin (5-HO-DMT), it has been used as an entheogen in South America. Slang terms include Five-methoxy, The power, and Toad venom. Chemistry 5-MeO-DMT was first synthesized in 1936, and in 1959 it was isolated as one of the psychoactive ingredients of '' Anadenanthera peregrina'' seeds used in preparing Yopo snuff. It was once believed to be a major component of the psychoactive effects of the snuff, although this has recently been shown to be unlikely, due to the limited or sometimes even non-existent quantity contained within the seeds, which instead achieve their psychoactivity from the ''O''-demethylated metabolite of 5-MeO-DMT, bufotenin. It is metabolized mainly by CYP2D6. Effects D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-MeO-DiBF
5-MeO-DiBF is a psychedelic that has been sold online as a designer drug and was first definitively identified in December 2015 by a forensic laboratory in Slovenia. It is thought to act as an agonist for the 5-HT1A and 5-HT2 family of serotonin receptors. It is related in structure to the psychedelic tryptamine derivative 5-MeO-DiPT, but with the indole nitrogen replaced by oxygen, making 5-MeO-DiBF a benzofuran derivative. It is several times less potent as a serotonin agonist than 5-MeO-DiPT and with relatively more activity at 5-HT1A, but still shows strongest effects at the 5-HT2 family of receptors. LEGAL STATUS. It is not controlled under the 1971 Convention on Psychotropic Substances, so thus it has a legal grey area in many countries of the world, but its consumption still could be persecuted under severe analogue acts or the intend of sell to human consumption. See also * Dimemebfe Dimemebfe (5-MeO-BFE) is a recreational drug and research chemical. It act ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Designer Drugs
A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Designer drugs include psychoactive substances that have been designated by the European Union as new psychoactive substances (NPS) as well as analogs of performance-enhancing drugs such as designer steroids. Some of these were originally synthesized by academic or industrial researchers in an effort to discover more potent derivatives with fewer side effects, and shorter duration (and possibly also because it is easier to apply for patents for new molecules) and were later co-opted for recreational use. Other designer drugs were prepared for the first time in clandestine laboratories. Because the efficacy and safety of these substances have not been thoroughly evaluated in animal and human trials, the use of some of these drugs may result i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Controlled Substances Act
The Controlled Substances Act (CSA) is the statute establishing federal government of the United States, federal drug policy of the United States, U.S. drug policy under which the manufacture, importation, possession, use, and distribution of certain substances is regulated. It was passed by the 91st United States Congress as Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970 and signed into law by President Richard Nixon. The Act also served as the national implementing legislation for the Single Convention on Narcotic Drugs. The legislation created five schedules (classifications), with varying qualifications for a substance to be included in each. Two federal agencies, the Drug Enforcement Administration (DEA) and the Food and Drug Administration (FDA), determine which substances are added to or removed from the various schedules, although the statute passed by Congress created the initial listing. Congress has sometimes scheduled other substances th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Agonist
A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin (5-hydroxytryptamine; 5-HT), a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors. Non-selective agonists Serotonergic psychedelics such as tryptamines (e.g., psilocybin, psilocin, , 5-MeO-DMT, bufotenin), lysergamides (e.g., , ergine ()), phenethylamines (e.g., mescaline, 2C-B, 25I-NBOMe), and amphetamines (e.g., , ) are non-selective agonists of serotonin receptors. Their hallucinogenic effects are specifically mediated by activation of the 5-HT2A receptor. Drugs that increase extracellular serotonin levels such as serotonin reuptake inhibitors (e.g., fluoxetine, venlafaxine), serotonin releasing agents (e.g., fenfluramine, ), and monoamine oxidase inhibitors (e.g., phenelzine, moclobemide) are indirect non-selective serotonin receptor agonists. They are used variously as antidepressants, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzofuran
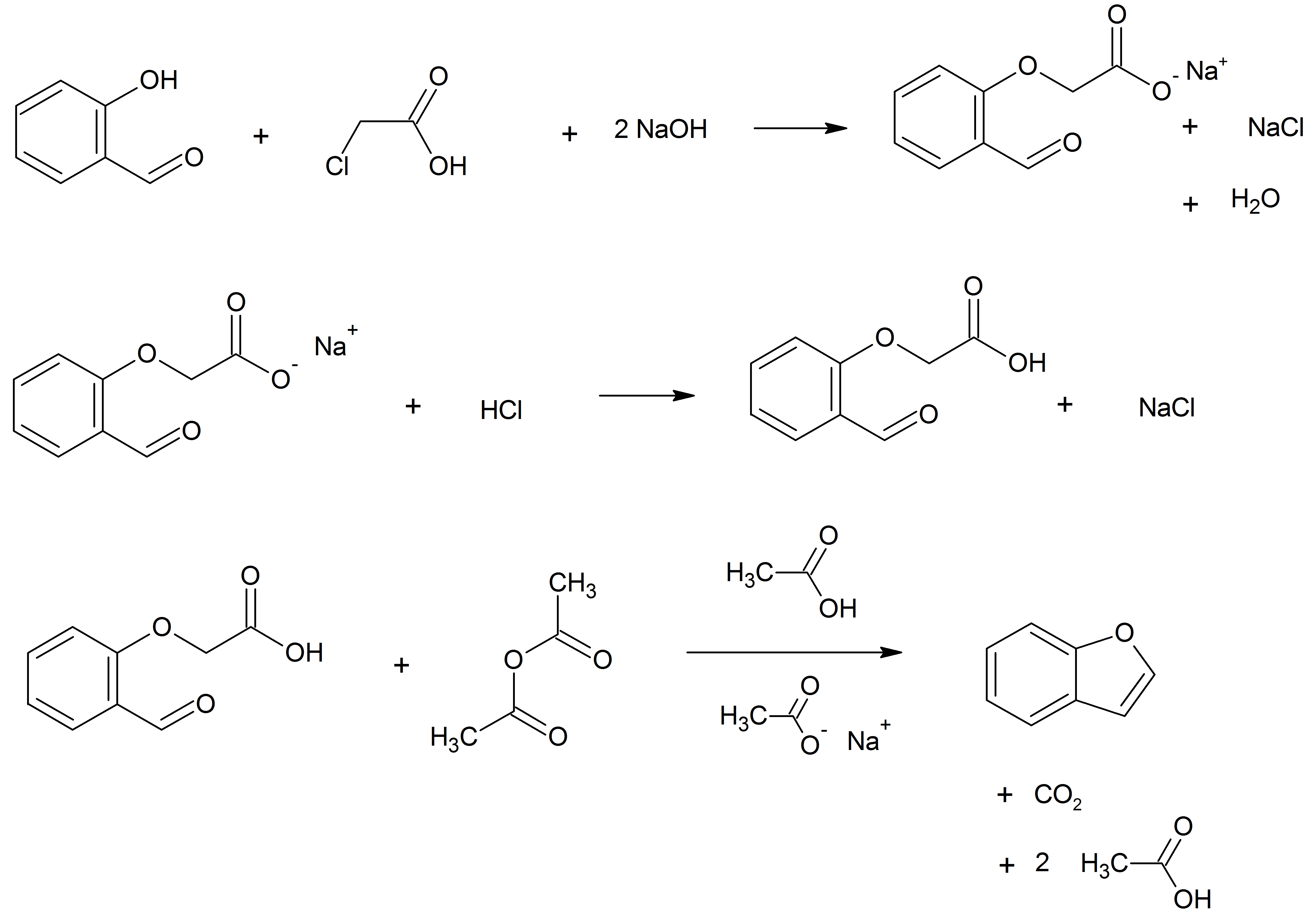
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : * Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px * Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioisostere
In medicinal chemistry, bioisosteres are chemical substituents or groups with similar physical or chemical properties which produce broadly similar biological properties to another chemical compound. In drug design, the purpose of exchanging one bioisostere for another is to enhance the desired biological or physical properties of a compound without making significant changes in chemical structure. The main use of this term and its techniques are related to pharmaceutical sciences. Bioisosterism is used to reduce toxicity, change bioavailability, or modify the activity of the lead compound, and may alter the metabolism of the lead. Examples Classical bioisosteres Classical bioisosterism was originally formulated by James Moir and refined by Irving Langmuir as a response to the observation that different atoms with the same valence electron structure had similar biological properties. For example, the replacement of a hydrogen atom with a fluorine atom at a site of metabolic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recreational Drug
Recreational drug use indicates the use of one or more psychoactive drugs to induce an altered state of consciousness either for pleasure or for some other casual purpose or pastime by modifying the perceptions and emotions of the user. When a psychoactive drug enters the user's body, it induces an intoxicating effect. Generally, recreational drugs are divided into three categories: depressants (drugs that induce a feeling of relaxation and calmness); stimulants (drugs that induce a sense of energy and alertness); and hallucinogens (drugs that induce perceptual distortions such as hallucination). In popular practice, recreational drug use generally is a tolerated social behaviour, rather than perceived as the medical condition of self-medication. However, heavy use of some drugs is socially stigmatized. Many people also use prescribed and controlled depressants such as opioids, as well as opiates and benzodiazepines. Common recreational drugs include caffeine, commonly foun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Research Chemical
Research chemicals are chemical substances used by scientists for medical and scientific research purposes. One characteristic of a research chemical is that it is for laboratory research use only; a research chemical is not intended for human or veterinary use. This distinction is required on the labels of research chemicals, and is what exempts them from regulation under parts 100-740 in Title 21 of the Code of Federal Regulations ( 21CFR). Background Pharmacological research chemicals Research chemicals are fundamental in the development of novel pharmacotherapies. Common medical laboratory uses include ''in vivo'' and animal testing to determine therapeutic value, toxicology testing by contract research organizations to determine drug safety, and analysis by drug test and forensic toxicology labs for the purposes of evaluating human exposure. Many pharmacologically active chemicals are sold online under the guise of "research chemicals," when in reality they are untested d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |