|
Dihydroimidazol-2-ylidene
Dihydroimidazol-2-ylidene is a hypothetical organic compound with formula C3H6N2. It would be a heterocyclic compound, formally derived from imidazolidine with two hydrogen atoms removed from carbon number 2, leaving two vacant chemical bonds — which makes it a carbene. Although carbenes in general are extremely short-lived, some derivatives of this compound are surprisingly stable, and form an important class of the persistent carbenes. They include the first stable carbenes postulated (but not isolated) by Hans-Werner Wanzlick around 1960. They also include an example of the (saturated) imidazolin-2-ylidene (carbene In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms. The term "carbene" ma ...) reported by A.J. Arduengo in 1995. References Carbenes Imidazolines Hypothetical chemical compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Persistent Carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole. Traditionally carbenes are viewed as so reactive that were only studied indirectly, such as by trapping reactions. This situation has changed dramatically with the emergence of persistent carbenes. Although they are fairly reactive substances, undergoing dimerization, many can be isolated as pure substances. Persistent carbenes tend to exist in the singlet. Their stability is only partly due to steric hindrance by bulky groups. Some singlet carbenes are thermodynamically stable and can be iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazolidine
Imidazolidine is a heterocyclic compound (CH2)2(NH)2CH2. The parent imidazolidine is lightly studied, but related compounds substituted on one or both nitrogen centers are more common. Generally, they are colorless, polar, basic compounds. Imidazolidines are cyclic 5-membered examples of the general class of aminals. Preparation Imidazolidines are traditionally prepared by condensation reaction of 1,2-diamines and aldehydes. Most commonly, one or both nitrogen center is substituted with an alkyl or benzyl (Bn) group:Ferm, R. J.; Riebsomer, J. L. From "The chemistry of the 2-imidazolines and imidazolidines" Chemical Reviews, 1954, 54, 593-613. :(CH2NBn)2 + PhCHO → (CH2NBn)2C(H)Ph + H2O The first unsubstituted imidazolidine synthesis was reported in 1952. Reactions Unsubstituted imidazolidines are often labile. The rings are susceptible to hydrolysis back to the diamine and the aldehyde. Formally, removal of the two hydrogens at carbon 2 (between the two nitrogens) w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
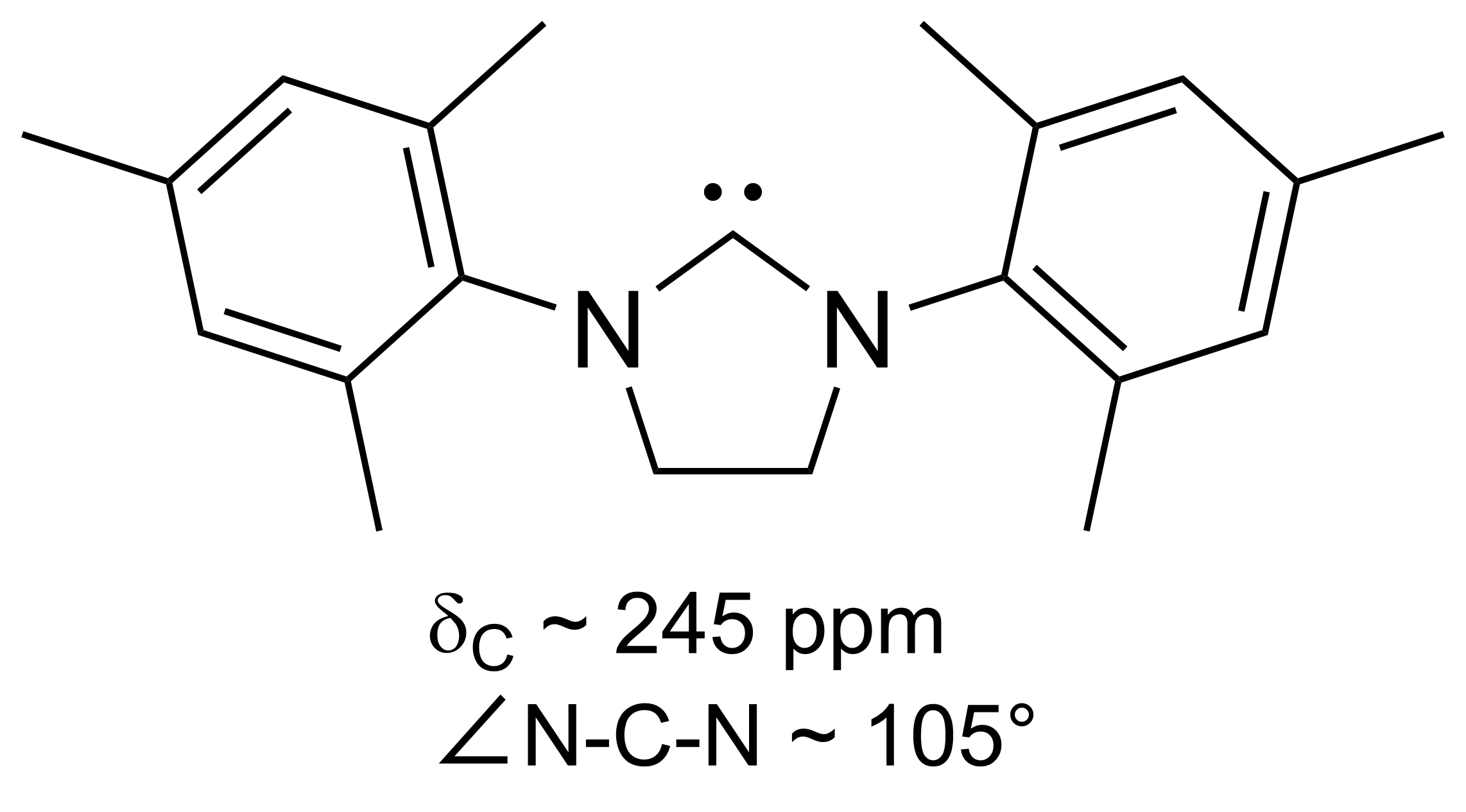
SIMes
SIMes (or H2Imes) is an ''N''-heterocyclic carbene. It is a white solid that dissolves in organic solvents. The compound is used as a ligand in organometallic chemistry. It is structurally related to the more common ligand IMes but with a saturated backbone (the S of SIMes indicates a saturated backbone). It is slightly more flexible and is a component in Grubbs II. It is prepared by alkylation of trimethylaniline by dibromoethane followed by ring closure and dehydrohalogenation. References {{organic-compound-stub Carbenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthony Joseph Arduengo, III
Anthony Joseph Arduengo III is Professor of the Practice at the Georgia Institute of Technology, Saxon Professor Emeritus of Chemistry at the University of Alabama, adjunct professor at the Institute for Inorganic Chemistry of Braunschweig University of Technology in Germany, and co-founder of the ''StanCE'' coalition for sustainable chemistry based on woody biomassXylochemistry. He is notable for his work on chemical compounds with unusual valency, especially in the field of stable carbene research. Early life Anthony "Bo" Arduengo was born in 1952 in Tampa, Florida. He grew up in the Atlanta, Georgia area. His father was a pressman and mechanic with the Atlanta Journal-Constitution and instilled his son with an interest and skill for all things mechanical and scientific. By the age of 16, he and his father had built his first car from miscellaneous parts. The car was registered as street-legal and road-worthy. With some re-engineering, the car was later fitted to run on alterna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthony Joseph Arduengo III
Anthony Joseph Arduengo III is Professor of the Practice at the Georgia Institute of Technology, Saxon Professor Emeritus of Chemistry at the University of Alabama, adjunct professor at the Institute for Inorganic Chemistry of Braunschweig University of Technology in Germany, and co-founder of the ''StanCE'' coalition for sustainable chemistry based on woody biomassXylochemistry. He is notable for his work on chemical compounds with unusual valency, especially in the field of stable carbene research. Early life Anthony "Bo" Arduengo was born in 1952 in Tampa, Florida. He grew up in the Atlanta, Georgia area. His father was a pressman and mechanic with the Atlanta Journal-Constitution and instilled his son with an interest and skill for all things mechanical and scientific. By the age of 16, he and his father had built his first car from miscellaneous parts. The car was registered as street-legal and road-worthy. With some re-engineering, the car was later fitted to run on al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. The strength of chemical bonds varies considerably; there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force and hydrogen bonding. Strong chemical bonding arises from the sharing or transfer of electrons between the participating atoms. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms. The term "carbene" may also refer to the specific compound , also called methylene, the parent hydride from which all other carbene compounds are formally derived. Carbenes are classified as either singlets or triplets, depending upon their electronic structure. Most carbenes are very short lived, although persistent carbenes are known. One well-studied carbene is dichlorocarbene , which can be generated ''in situ'' from chloroform and a strong base. Structures and bonding The two classes of carbenes are singlet and triplet carbenes. Singlet carbenes are spin-paired. In the language of valence bond theory, the molecule adopts an sp2 hybrid structure. Triplet carbenes have two unpaired electrons. Most carbenes have a nonlinear triplet ground state, e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hans-Werner Wanzlick
Hans-Werner Wanzlick (1917-1988) was a German chemist. A Professor of chemistry at the Berlin Technical University he is notable for work on persistent carbenes and for proposing the Wanzlick equilibrium between saturated imidazolin-2-ylidenes and their dimer Dimer may refer to: * Dimer (chemistry), a chemical structure formed from two similar sub-units ** Protein dimer, a protein quaternary structure ** d-dimer * Dimer model, an item in statistical mechanics, based on ''domino tiling'' * Julius Dimer ...s — which he called "''das doppelte Lottchen''", after a 1949 novel by Erich Kästner about a pair of mischievous twins. Anthony J. Arduengo, III (1999) ''Looking for Stable Carbenes: The Difficulty in Starting Anew''. Accounts of Chemical Research, volume 32, number 11, pages 913–921. References * Hans-Werner Wanzlick (1958), letter to Linus Pauling (in German). Linus Pauling's Correspondence, item #444.6. Technical University of Berlin faculty 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angewandte Chemie
''Angewandte Chemie'' (, meaning "Applied Chemistry") is a weekly peer-reviewed scientific journal that is published by Wiley-VCH on behalf of the German Chemical Society (Gesellschaft Deutscher Chemiker). Publishing formats include feature-length reviews, short highlights, research communications, minireviews, essays, book reviews, meeting reviews, correspondences, corrections, and obituaries. This journal contains review articles covering all aspects of chemistry. According to the ''Journal Citation Reports'', the journal had a 2021 impact factor of 16.823. Editions The journal appears in two editions with separate volume and page numbering: a German edition, ''Angewandte Chemie'' ( (print), (online)), and a fully English-language edition, ''Angewandte Chemie International Edition'' ( (print), (online)). The editions are identical in content with the exception of occasional reviews of German-language books or German translations of IUPAC recommendations. Business model ''A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemische Berichte
''Chemische Berichte'' (usually abbreviated as ''Ber.'' or ''Chem. Ber.'') was a German-language scientific journal of all disciplines of chemistry founded in 1868. It was one of the oldest scientific journals in chemistry, until it merged with ''Recueil des Travaux Chimiques des Pays-Bas'' to form ''Chemische Berichte/Recueil'' in 1997. ''Chemische Berichte/Recueil'' was then merged with other European journals in 1998 to form ''European Journal of Inorganic Chemistry''. History Founded in 1868 as ''Berichte der Deutschen Chemischen Gesellschaft'' (, CODEN BDCGAS), it operated under this title until 1928 (Vol. 61). The journal was then split into: * ''Berichte der Deutschen Chemischen Gesellschaft, A: Vereins-Nachrichten'' (, CODEN BDCAAS), and * ''Berichte der Deutschen Chemischen Gesellschaft, B: Abhandlungen'' (, CODEN BDCBAD). Vol. 78 and 79 (1945–1946) were omitted and not published due to World War II. The journal was renamed ''Chemische Berichte'' (, CODEN CHBEAM) in 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |