|
Dibromophenol
Dibromophenols form a group of aromatic chemical compounds which are both phenols and bromobenzenes. The structure consists of a benzene ring with an attached hydroxy group (-OH) and two bromine atoms (-Br) as substituents. There are six structural isomer In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a chemical compound, compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct chemical bond, b ...s, each with the molecular formula C6H4Br2O, which differ by arrangement of the substituents. References {{reflist Bromoarenes Phenols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,4-Dibromophenol
2,4-Dibromophenol is a brominated derivative of phenol with the molecular formula C6H4Br2O. It belongs to the bromobenzenes, which are organic compounds containing bromine atoms attached to a benzene ring. Properties At room temperature, 2,4-dibromophenol is a solid with needle-like crystals. It melts at and boils at . it has a molecular weight of 251.905 g/mol. It is soluble in water, ethanol, ether and benzene and slightly soluble in carbon tetrachloride. Occurrence 2,4-Dibromophenol is found in certain molluscs and crustaceans, as well as the acorn worm The acorn worms or Enteropneusta are a Hemichordata, hemichordate class of invertebrates consisting of one order of the same name. The closest non-hemichordate relatives of the Enteropneusta are the echinoderms. There are 111 known species of aco ... '' Saccoglossus bromophenolosus'', which is named after it. References {{DEFAULTSORT:Dibromophenol, 2,4- Phenols Bromoarenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning. Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
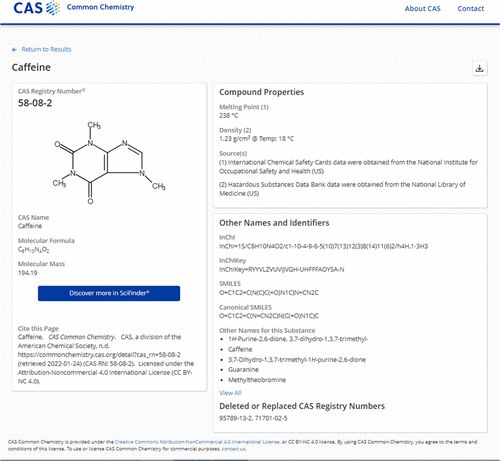
CAS Number
A CAS Registry Number (also referred to as CAS RN or informally CAS Number) is a unique identification number assigned by the Chemical Abstracts Service (CAS), US to every chemical substance described in the open scientific literature. It includes all substances described from 1957 through the present, plus some substances from as far back as the early 1800s. It is a chemical database that includes organic and inorganic compounds, minerals, isotopes, alloys, mixtures, and nonstructurable materials (UVCBs, substances of unknown or variable composition, complex reaction products, or biological origin). CAS RNs are generally serial numbers (with a check digit), so they do not contain any information about the structures themselves the way SMILES and InChI strings do. The registry maintained by CAS is an authoritative collection of disclosed chemical substance information. It identifies more than 182 million unique organic and inorganic substances and 68 million protein and DNA seq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GHS Hazard Statements
Hazard statements form part of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). They are intended to form a set of standardized phrases about the hazards of chemical substances and mixtures that can be translated into different languages. As such, they serve the same purpose as the well-known R-phrases, which they are intended to replace. Hazard statements are one of the key elements for the labelling of containers under the GHS, along with: *an identification of the product *one or more hazard pictograms (''where necessary'') *a signal word – either Danger or Warning – where necessary * precautionary statements, indicating how the product should be handled to minimize risks to the user (as well as to other people and the general environment) *the identity of the supplier (who might be a manufacturer or importer). Each hazard statement is designated a code, starting with the letter H and followed by three digits. Statements which correspond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GHS Hazard Pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS). Two sets of pictograms are included within the GHS: one for the labelling of containers and for workplace hazard warnings, and a second for use during the transport of dangerous goods. Either one or the other is chosen, depending on the target audience, but the two are not used together. The two sets of pictograms use the same symbols for the same hazards, although certain symbols are not required for transport pictograms. Transport pictograms come in wider variety of colors and may contain additional information such as a subcategory number. Hazard pictograms are one of the key elements for the labelling of containers under the GHS, along with:Part 1, section 1.4.10.5.2, GHS Rev.2 *an identification of the product; *a signal word – either Danger or Warning – where necessary * hazard statements, indicating the nature and degree of the risks posed by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting Point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa. When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the melting point." Examples For most substances, melting and freezing points are approximately equal. For example, the melting point ''and'' freezing point of mercury is . How ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical State
In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose–Einstein condensates (in extreme cold), neutron-degenerate matter (in extreme density), and quark–gluon plasma (at extremely high energy). For a complete list of all exotic states of matter, see the list of states of matter. Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume (assuming no change in temperature or air pressure) and shape, with component particles (atoms, molecules or ions) close together and fixed into place. Matter in the liquid state maintains a fixed volume (assuming no change in temperature or air pressure), but has a variable shape that adapts to fit its container. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Mass
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance which is the number of moles in that sample, measured in moles. The molar mass is a bulk, not molecular, property of a substance. The molar mass is an ''average'' of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities. The molecular mass and formula mass are commonly used as a synonym of molar mass, particularly for molecular compounds; however, the most authoritative sources define it differently. The difference is that molecular mass is the mass of one specific particle or molecul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include Subscript and superscript, subscripts and superscripts. A chemical formula is not a chemical nomenclature, chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called ''empirical formulae'', which use letters and numbers ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PubChem
PubChem is a database of chemical molecules and their activities against biological assays. The system is maintained by the National Center for Biotechnology Information (NCBI), a component of the National Library of Medicine, which is part of the United States National Institutes of Health (NIH). PubChem can be accessed for free through a web user interface. Millions of compound structures and descriptive datasets can be freely downloaded via FTP. PubChem contains multiple substance descriptions and small molecules with fewer than 100 atoms and 1,000 bonds. More than 80 database vendors contribute to the growing PubChem database. History PubChem was released in 2004 as a component of the Molecular Libraries Program (MLP) of the NIH. As of November 2015, PubChem contains more than 150 million depositor-provided substance descriptions, 60 million unique chemical structures, and 225 million biological activity test results (from over 1 million assay experiments performed on more t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bonding within the molecule is also shown, either explicitly or implicitly. Unlike other chemical formula types, which have a limited number of symbols and are capable of only limited descriptive power, structural formulas provide a more complete geometric representation of the molecular structure. For example, many chemical compounds exist in different isomeric forms, which have different enantiomeric structures but the same molecular formula. There are multiple types of ways to draw these structural formulas such as: Lewis Structures, condensed formulas, skeletal formulas, Newman projections, Cyclohexane conformations, Haworth projections, and Fischer projections. Several systematic chemical naming formats, as in chemical databases, are used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)