|
Decamethylsilicocene
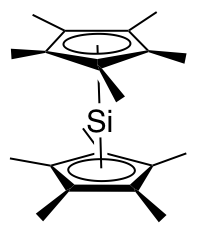
Decamethylsilicocene, (C5Me5)2Si, is a group 14 sandwich compound. It is an example of a main-group cyclopentadienyl complex; these molecules are related to metallocenes but contain p-block elements as the central atom. It is a colorless, air sensitive solid that sublimes under vacuum. Synthesis The first synthesis of decamethylsilicocene was reported by Jutzi and coworkers in 1986. It involved reduction of bis(pentamethylcyclopentadienyl)silicon(IV) dichloride with two equivalents of sodium naphthalenide to generate decamethylsilicocene, naphthalene, and sodium chloride. Generation of the sterically crowded bis(pentamethylcyclopentadienyl)silicon(IV) dichloride required several steps, beginning with double deprotonation of (C5Me4H)2SiCl2 using ''tert''-butyllithium, followed by treatment of the resultant (C5Me4Li)2SiCl2 with methyl iodide. Decamethylsilicocene is soluble in aprotic solvents such as hexane, benzene, and chlorinated solvents. Molecular weight determinations sho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decamethylsilicocene
Decamethylsilicocene, (C5Me5)2Si, is a group 14 sandwich compound. It is an example of a main-group cyclopentadienyl complex; these molecules are related to metallocenes but contain p-block elements as the central atom. It is a colorless, air sensitive solid that sublimes under vacuum. Synthesis The first synthesis of decamethylsilicocene was reported by Jutzi and coworkers in 1986. It involved reduction of bis(pentamethylcyclopentadienyl)silicon(IV) dichloride with two equivalents of sodium naphthalenide to generate decamethylsilicocene, naphthalene, and sodium chloride. Generation of the sterically crowded bis(pentamethylcyclopentadienyl)silicon(IV) dichloride required several steps, beginning with double deprotonation of (C5Me4H)2SiCl2 using ''tert''-butyllithium, followed by treatment of the resultant (C5Me4Li)2SiCl2 with methyl iodide. Decamethylsilicocene is soluble in aprotic solvents such as hexane, benzene, and chlorinated solvents. Molecular weight determinations sho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silylene
Silylene is a chemical compound with the formula SiH2. It is the silicon analog of methylene, the simplest carbene. Silylene is a stable molecule as a gas but rapidly reacts in a bimolecular manner when condensed. Unlike carbenes, which can exist in the singlet or triplet state, silylene (and all of its derivatives) are singlets. Silylenes are formal derivatives of silylene with its hydrogens replaced by other substituents. Most examples feature amido (NR2) or alkyl/aryl groups. Silylenes have been proposed as reactive intermediates. They are carbene analogs. Synthesis and properties Silylenes are generally synthesized by thermolysis or photolysis of polysilanes, by silicon atom reactions ( insertion, addition or abstraction), by pyrolysis of silanes, or by reduction of 1,1-dihalosilane. It has long been assumed that the conversion of metallic Si to tetravalent silicon compounds proceeds via silylene intermediates: :Si + Cl2 → SiCl2 :SiCl2 + Cl2 → SiCl4 Similar cons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-heterocyclic Carbene
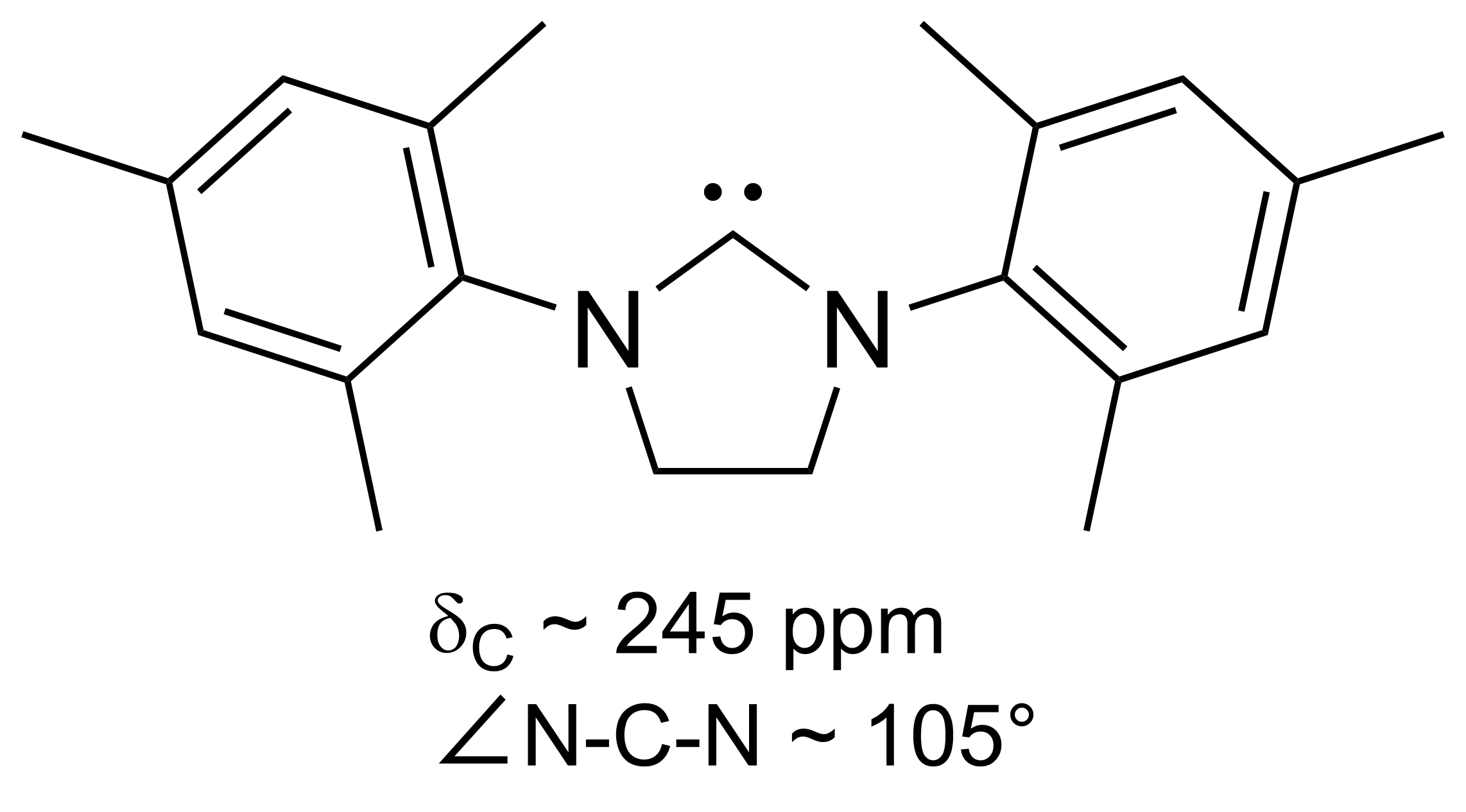
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole. Traditionally carbenes are viewed as so reactive that were only studied indirectly, such as by trapping reactions. This situation has changed dramatically with the emergence of persistent carbenes. Although they are fairly reactive substances, undergoing dimerization, many can be isolated as pure substances. Persistent carbenes tend to exist in the singlet. Their stability is only partly due to steric hindrance by bulky groups. Some singlet carbenes are thermodynamically stable and can be iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brookhart's Acid
Brookhart's acid is the salt of the diethyl ether oxonium ion and tetrakis ,5-bis(trifluoromethyl)phenylorate (BAr′4). It is a colorless solid, used as a strong acid. The compound was first reported by Volpe, Grant, and Brookhart in 1992. Preparation This compound is prepared by treatment of NaBAr′4 in diethyl ether (Et2O) with hydrogen chloride: : NaBAr′4 + HCl + 2 Et2O → (OEt2)2sup>+ + NaCl NaBAr′4 is soluble in diethyl ether, whereas sodium chloride is not. Precipitation of sodium chloride thus drives the formation of the oxonium acid compound, which is isolable as a solid. Structure and properties The acid crystallizes as a white, hygroscopic crystalline solid. NMR and elemental analysis showed that the crystal contains two equivalents of diethyl ether. In solution, the compound slowly degrades to ''m''-C6H3(CF3)2 and BAr′3. (OEt2)2B(C6F5)4] is a related compound with a slightly different weakly coordinating anion; it was first reported in 2000. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HOMO/LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontier orbitals'', such as in the frontier molecular orbital theory. Gap The energy difference between the HOMO and LUMO is ''the HOMO–LUMO gap''. Its size can be used to predict the strength and stability of transition metal complexes, as well as the colors they produce in solution.Griffith, J. S. and L. E. Orgel"Ligand Field Theory" ''Q. Rev. Chem. Soc.'' 1957, 11, 381–383. As a rule of thumb, the larger a compound's HOMO-LUMO gap, the more stable the compound. Semiconductors The HOMO level is to organic semiconductors roughly what the maximum valence band is to inorganic semiconductors and quantum dots. The same analogy can be made between the LUMO level and the conduction band minimum.Bredas, J,-L"Mind the gap!" ''Mater. Horiz.'' 201 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decamethylferrocene
Decamethylferrocene or bis(pentamethylcyclopentadienyl)iron(II) is a chemical compound with formula or . It is a sandwich compound, whose molecule has an iron(II) cation attached by coordination bonds between two pentamethylcyclopentadienyl anions (, ). It can also be viewed as a derivative of ferrocene, with a methyl group replacing each hydrogen atom of its cyclopentadienyl rings. The name and formula are often abbreviated to DmFc, or . This compound is a yellow crystalline solid that is used in chemical laboratories as a weak reductant. The iron(II) core is easily oxidized to iron(III), yielding the monovalent cation decamethylferrocenium, and even to higher oxidation states. Preparation Decamethylferrocene is prepared in the same manner as ferrocene from pentamethylcyclopentadiene. This method can be used to produce other decamethylcyclopentadienyl sandwich compounds. :2 Li(C5Me5) + FeCl2 → Fe(C5Me5)2 + 2 LiCl The product can be purified by sublimation. has stagg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bent Metallocene
In organometallic chemistry, bent metallocenes are a subset of metallocenes. In bent metallocenes, the ring systems coordinated to the metal are not parallel, but are tilted at an angle. A common example of a bent metallocene is Cp2TiCl2. Several reagents and much research is based on bent metallocenes. Synthesis Like regular metallocenes, bent metallocenes are synthesized by a variety of methods but most typically by reaction of sodium cyclopentadienide with the metal halide. This method applies to the synthesis of the bent metallocene dihalides of titanium, zirconium, hafnium, and vanadium: :2 NaC5H5 + TiCl4 → (C5H5)2TiCl2 + 2 NaCl In the earliest work in this area, Grignard reagents were used to deprotonate the cyclopentadiene. Niobocene dichloride, featuring Nb(IV), is prepared via a multistep reaction that begins with a Nb(V) precursor: :NbCl5 + 6 NaC5H5 → 5 NaCl + (C5H5)4Nb + organic products :(C5H5)4Nb + 2 HCl + 0.5 O2) → 2O">sub>2Ol2 + 2 C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |