|
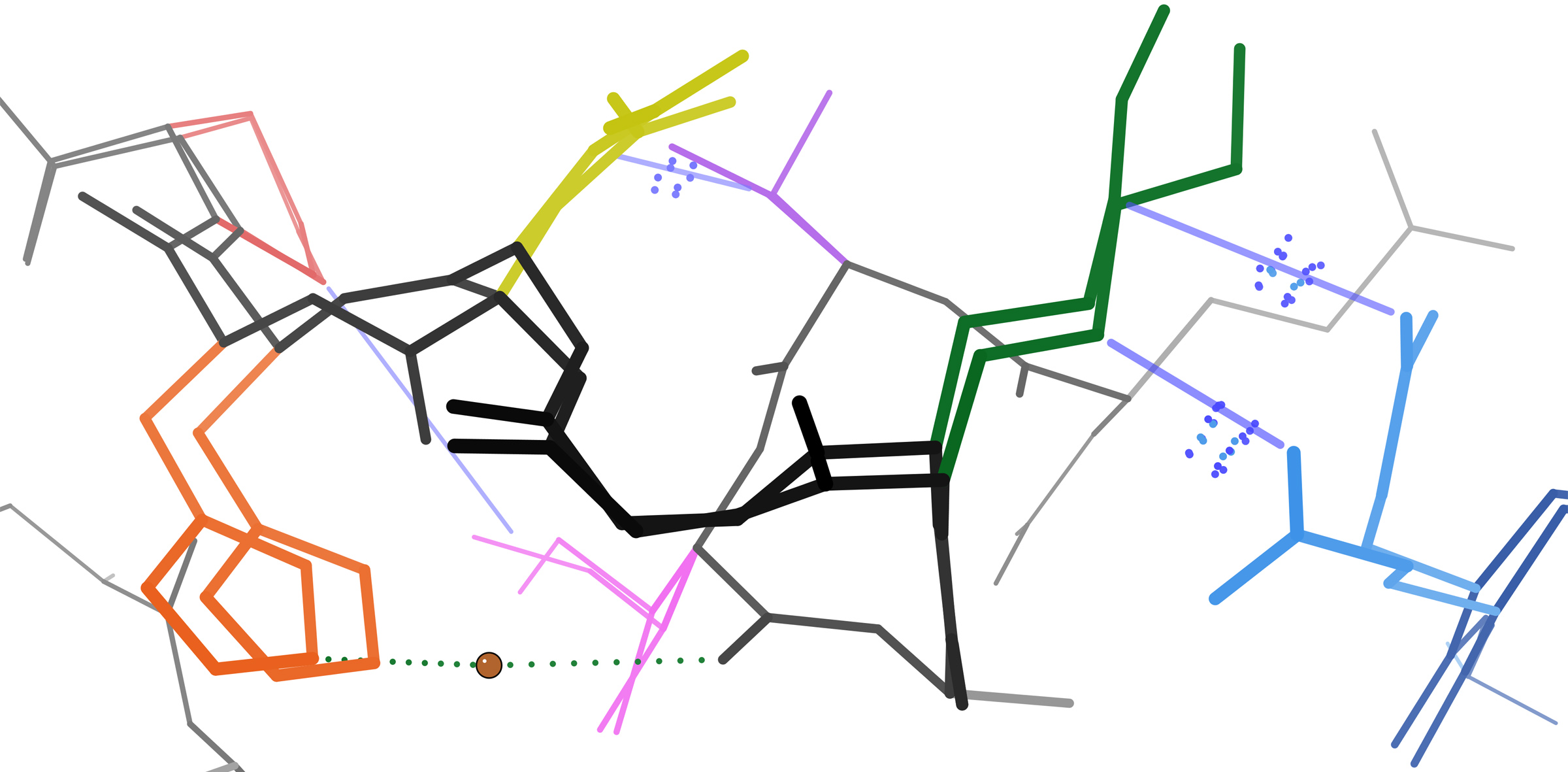
Database Of Protein Conformational Diversity
The Database of protein conformational diversity (PCDB) is a database of diversity of protein tertiary structures within protein domains as determined by X-ray crystallography#Biological macromolecular crystallography, X-ray crystallography. Proteins are inherently Protein motions, flexible and this database collects information on this subject for use in molecular research. It uses the CATH, CATH database as a source of structures for each protein and reports the range of differences in the structures based on their superposition and reports a maximum RMSD. The interface for the database allows researchers to find proteins with a range of conformational flexibility allowing them to find highly flexible proteins for example. The database is run and maintained by a group of researchers based at the Universidad Nacional de Quilmes in Argentina. See also *Crystallography *Protein structure References External links * Biological databases Protein structure Crystallographic da ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Database
In computing, a database is an organized collection of data stored and accessed electronically. Small databases can be stored on a file system, while large databases are hosted on computer clusters or cloud storage. The design of databases spans formal techniques and practical considerations, including data modeling, efficient data representation and storage, query languages, security and privacy of sensitive data, and distributed computing issues, including supporting concurrent access and fault tolerance. A database management system (DBMS) is the software that interacts with end users, applications, and the database itself to capture and analyze the data. The DBMS software additionally encompasses the core facilities provided to administer the database. The sum total of the database, the DBMS and the associated applications can be referred to as a database system. Often the term "database" is also used loosely to refer to any of the DBMS, the database system or an appli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single amino acid monomer may also be called a ''residue'' indicating a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reactions, in which the amino acids lose one water molecule per reaction in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their three-dimensional structure. This is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Universidad Nacional De Quilmes
The National University of Quilmes ( es, Universidad Nacional de Quilmes, UNQui) is an Argentine national university and the most important one in the Quilmes area. The National University of Quilmes was founded on October 23, 1989. Located in Bernal ( Quilmes County), it serves the Southern Buenos Aires Metropolitan Area, home to three million people and 20% of the country's industrial establishments. The UNQ has over thirty thousand students, distributed among its undergraduate courses and graduate courses of study. The university maintains 18 undergraduate programs (including seven through its virtual university program established in 1998), as well as four master's degree programs and two doctorates (Applied Sciences and Social Sciences). The university's stated mission is to teach in an environment of equality and diversity. Its essential functions are teaching, research, extension courses, human resources formation, technological development, productive innovation and cultu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Tertiary Structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may interact and bond in a number of ways. The interactions and bonds of side chains within a particular protein determine its tertiary structure. The protein tertiary structure is defined by its atomic coordinates. These coordinates may refer either to a protein domain or to the entire tertiary structure.Branden C. and Tooze J. "Introduction to Protein Structure" Garland Publishing, New York. 1990 and 1991. A number of tertiary structures may fold into a quaternary structure.Kyte, J. "Structure in Protein Chemistry." Garland Publishing, New York. 1995. History The science of the tertiary structure of proteins has progressed from one of hypothesis to one of detailed definition. Although Emil Fischer had suggested proteins were made of polypeptid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer aft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Motions
Proteins are generally thought to adopt unique structures determined by their amino acid sequences. However, proteins are not strictly static objects, but rather populate ensembles of (sometimes similar) conformations. Transitions between these states occur on a variety of length scales (tenths of Å to nm) and time scales (ns to s), and have been linked to functionally relevant phenomena such as allosteric signaling and enzyme catalysis. The study of protein dynamics is most directly concerned with the transitions between these states, but can also involve the nature and equilibrium populations of the states themselves. These two perspectives—kinetics and thermodynamics, respectively—can be conceptually synthesized in an "energy landscape" paradigm: highly populated states and the kinetics of transitions between them can be described by the depths of energy wells and the heights of energy barriers, respectively. Local flexibility: atoms and residues Portions of protein s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CATH
The CATH Protein Structure Classification database is a free, publicly available online resource that provides information on the evolutionary relationships of protein domains. It was created in the mid-1990s by Professor Christine Orengo and colleagues including Janet Thornton and David Jones, and continues to be developed by the Orengo group at University College London. CATH shares many broad features with the SCOP resource, however there are also many areas in which the detailed classification differs greatly. Hierarchical organization Experimentally-determined protein three-dimensional structures are obtained from the Protein Data Bank and split into their consecutive polypeptide chains, where applicable. Protein domains are identified within these chains using a mixture of automatic methods and manual curation. The domains are then classified within the CATH structural hierarchy: at the Class (C) level, domains are assigned according to their secondary structure content, i. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RMSD
The root-mean-square deviation (RMSD) or root-mean-square error (RMSE) is a frequently used measure of the differences between values (sample or population values) predicted by a model or an estimator and the values observed. The RMSD represents the square root of the second sample moment of the differences between predicted values and observed values or the quadratic mean of these differences. These deviations are called '' residuals'' when the calculations are performed over the data sample that was used for estimation and are called ''errors'' (or prediction errors) when computed out-of-sample. The RMSD serves to aggregate the magnitudes of the errors in predictions for various data points into a single measure of predictive power. RMSD is a measure of accuracy, to compare forecasting errors of different models for a particular dataset and not between datasets, as it is scale-dependent. RMSD is always non-negative, and a value of 0 (almost never achieved in practice) would ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallography
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The word "crystallography" is derived from the Greek word κρύσταλλος (''krystallos'') "clear ice, rock-crystal", with its meaning extending to all solids with some degree of transparency, and γράφειν (''graphein'') "to write". In July 2012, the United Nations recognised the importance of the science of crystallography by proclaiming that 2014 would be the International Year of Crystallography. denote a direction vector (in real space). * Coordinates in ''angle brackets'' or ''chevrons'' such as <100> denote a ''family'' of directions which are related by symmetry operations. In the cubic crystal system for example, would mean 00 10 01/nowiki> or the negative of any of those directions. * Miller indices in ''parentheses'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biological Databases
Biological databases are libraries of biological sciences, collected from scientific experiments, published literature, high-throughput experiment technology, and computational analysis. They contain information from research areas including genomics, proteomics, metabolomics, microarray gene expression, and phylogenetics. Information contained in biological databases includes gene function, structure, localization (both cellular and chromosomal), clinical effects of mutations as well as similarities of biological sequences and structures. Biological databases can be classified by the kind of data they collect (see below). Broadly, there are molecular databases (for sequences, molecules, etc.), functional databases (for physiology, enzyme activities, phenotypes, ecology etc), taxonomic databases (for species and other taxonomic ranks), images and other media, or specimens (for museum collections etc.) Databases are important tools in assisting scientists to analyze and explain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |