|
Dock6
Dock6 (Dedicator of cytokinesis 6), also known as Zir1 is a large (~200 kDa) protein involved in intracellular signalling networks. It is a member of the DOCK-C subfamily of the DOCK family of guanine nucleotide exchange factors which function as activators of small G proteins. Discovery Dock6 was identified as one of a family of proteins which share high sequence similarity with Dock180 Dock180, (Dedicator of cytokinesis) also known as DOCK1, is a large (~180 kDa) protein involved in intracellular signalling networks. It is the mammalian ortholog of the ''C. elegans'' protein CED-5 and belongs to the DOCK family of Guanine nucl ..., the archetypal member of the DOCK family. It has a similar domain arrangement to other DOCK proteins, with a DHR1 domain known in other proteins to bind phospholipids, and a DHR2 domain containing the GEF activity. Function There is currently very little information about the cellular role of this protein. However, Dock6 has been reporte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid resid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intracellular
This glossary of biology terms is a list of definitions of fundamental terms and concepts used in biology, the study of life and of living organisms. It is intended as introductory material for novices; for more specific and technical definitions from sub-disciplines and related fields, see Glossary of genetics, Glossary of evolutionary biology, Glossary of ecology, and Glossary of scientific naming, or any of the organism-specific glossaries in :Glossaries of biology. A B C D E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
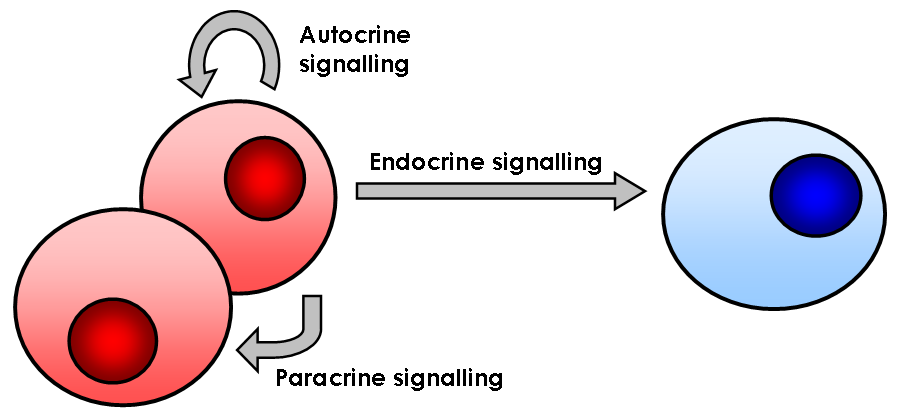
Cell Signalling
In biology, cell signaling (cell signalling in British English) or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellular life in prokaryotes and eukaryotes. Signals that originate from outside a cell (or extracellular signals) can be physical agents like mechanical pressure, voltage, temperature, light, or chemical signals (e.g., small molecules, peptides, or gas). Cell signaling can occur over short or long distances, and as a result can be classified as autocrine, juxtacrine, intracrine, paracrine, or endocrine. Signaling molecules can be synthesized from various biosynthetic pathways and released through passive or active transports, or even from cell damage. Receptors play a key role in cell signaling as they are able to detect chemical signals or physical stimuli. Receptors are generally proteins located on the cell surface or within the inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DOCK (protein)
DOCK (Dedicator of cytokinesis) is a family of related proteins involved in intracellular signalling networks. DOCK family members contain a RhoGEF domain to function as guanine nucleotide exchange factors to promote GDP release and GTP binding to specific Small GTPases of the Rho family (e.g., Rac and Cdc42), leading to their activation since Rho proteins are inactive when bound to GDP but active when bound to GTP. DOCK family proteins are categorised into four subfamilies based on their sequence homology: *DOCK-A subfamily **Dock180 (also known as Dock1) **Dock2 ** Dock5 *DOCK-B subfamily ** Dock3 (also known as MOCA and PBP) ** Dock4 *DOCK-C subfamily (also known as Zir subfamily) **Dock6 (also known as Zir1) ** Dock7 (also known as Zir2) ** Dock8 (also known as Zir3) *DOCK-D subfamily (also known as Zizimin subfamily) **Dock9 (also known as Zizimin1) ** Dock10 (also known as Zizimin3) **Dock11 Dock11 (Dedicator of cytokinesis), also known as Zizimin2, is a large (~240 kDa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanine Nucleotide Exchange Factor
Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase. Function Guanine nucleotide exchange factors (GEFs) are proteins or protein domains involved in the activation of small GTPases. Small GTPases act as molecular switches in intracellular signaling pathways and have many downstream targets. The most well-known GTPases comprise the Ras superfamily and are involved in essential cell processes such as cell differentiation and proliferation, cytoskeletal organization, vesicle trafficking, and nuclear transport. GTPases are active when bound to GTP and inactive when bound to GDP, allowing their activity to be regulated by GEFs and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein complexes. The latter class of complexes is made up of ''alpha'' (α), ''beta'' (β) and ''gamma'' (γ) subunits. In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex . Heterotrimeric G proteins located within the cell a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sequence Similarity
Sequence homology is the biological homology between DNA, RNA, or protein sequences, defined in terms of shared ancestry in the evolutionary history of life. Two segments of DNA can have shared ancestry because of three phenomena: either a speciation event (orthologs), or a duplication event (paralogs), or else a horizontal (or lateral) gene transfer event (xenologs). Homology among DNA, RNA, or proteins is typically inferred from their nucleotide or amino acid sequence similarity. Significant similarity is strong evidence that two sequences are related by evolutionary changes from a common ancestral sequence. Alignments of multiple sequences are used to indicate which regions of each sequence are homologous. Identity, similarity, and conservation The term "percent homology" is often used to mean "sequence similarity”, that is the percentage of identical residues (''percent identity''), or the percentage of residues conserved with similar physicochemical properties (' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dock180
Dock180, (Dedicator of cytokinesis) also known as DOCK1, is a large (~180 kDa) protein involved in intracellular signalling networks. It is the mammalian ortholog of the ''C. elegans'' protein CED-5 and belongs to the DOCK family of Guanine nucleotide exchange factors (GEFs). Discovery Dock180 was identified, using a far-western blotting approach, as a binding partner of the adaptor protein Crk that was able to induce morphological changes in 3T3 fibroblasts. Subsequently it was reported that Dock180 was able to activate the small GTP-binding protein (G protein) Rac1 and this was later shown to happen via its ability to act as a GEF. Structure and function Dock180 is part of a large class of proteins (GEFs) which contribute to cellular signalling events by activating small G proteins. In their resting state G proteins are bound to Guanosine diphosphate (GDP) and their activation requires the dissociation of GDP and binding of guanosine triphosphate (GTP). GEFs activate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DHR1 Domain
DHR1 (DOCK homology region 1), also known as CZH1 or Docker1, is a protein domain of approximately 200–250 amino acids that is present in the DOCK family of signalling proteins. This domain binds phospholipids and so may assist in recruitment to cellular membranes. There is evidence that this domain may also mediate protein–protein interaction Protein–protein interactions (PPIs) are physical contacts of high specificity established between two or more protein molecules as a result of biochemical events steered by interactions that include electrostatic forces, hydrogen bonding and th ...s. References Further reading * * {{refend Protein domains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phospholipids
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typically have omega-3 fatty acids EPA and DHA integrated as part of the phospholipid molecule. The phosphate group can be modified with simple organic molecules such as choline, ethanolamine or serine. Phospholipids are a key component of all cell membranes. They can form lipid bilayers because of their amphiphilic characteristic. In eukaryotes, cell membranes also contain another class of lipid, sterol, interspersed among the phospholipids. The combination provides fluidity in two dimensions combined with mechanical strength against rupture. Purified phospholipids are produced commercially and have found applications in nanotechnology and materials science. The first phospholipid identified in 1847 as such in biological tissues was leci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DHR2 Domain
DHR2 (DOCK homology region 2), also known as CZH2 or Docker2, is a protein domain of approximately 450-550 amino acids that is present in the DOCK A dock (from Dutch language, Dutch ''dok'') is the area of water between or next to one or a group of human-made structures that are involved in the handling of boats or ships (usually on or near a shore) or such structures themselves. The ex ... family of proteins. This domain functions as a guanine nucleotide exchange factor (GEF) domain for small G proteins of the Rho family. DHR2 domains bear no significant similarity to the well described DH domain (Dbl homologous domain) present in other RhoGEFs such as Vav, P-Rex and TRIO. Indeed, the most divergent mammalian DHR2 domains share only 16-17% sequence similarity. References Further reading * * *{{cite journal , vauthors=Lu M, Kinchen JM, Rossman KL, title=GEF A Steric-inhibition model for regulation of nucleotide exchange via the Dock180 family of GEFs, journal=Curr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |