|
D-Amino Acid
D-Amino acids are amino acids where the stereogenic carbon alpha to the amino group has the D-configuration. For most naturally-occurring amino acids, this carbon has the L-configuration. D-Amino acids are occasionally found in nature as residues in proteins. They are formed from ribosomally-derived D-amino acid residues. Amino acids, as components of peptides, peptide hormones, structural and immune proteins, are the most important bioregulators involved in all life processes along with nucleic acids, carbohydrates and lipids. "Environmental D-amino acids are thought to be derived from organic diagenesis such as racemization and release from bacterial cell walls and even from microbial production." Discovery Their discovery was in the 1950s. "Auclair and Patton (1950) first reported their presence in the blood of insects and mollusks" Furthermore, they also have been identified in various mammalian tissues. The two major types of D-amino acids synthesized in and by mamm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram-positive
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall. Gram-positive bacteria take up the crystal violet stain used in the test, and then appear to be purple-coloured when seen through an optical microscope. This is because the thick peptidoglycan layer in the bacterial cell wall retains the stain after it is washed away from the rest of the sample, in the decolorization stage of the test. Conversely, gram-negative bacteria cannot retain the violet stain after the decolorization step; alcohol used in this stage degrades the outer membrane of gram-negative cells, making the cell wall more porous and incapable of retaining the crystal violet stain. Their peptidoglycan layer is much thinner and sandwiched between an inner cell membrane and a bacterial outer membrane, causing them to take up the counterstain (saf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacitracin
Bacitracin is a polypeptide antibiotic. It is a mixture of related cyclic peptides produced by ''Bacillus licheniformis'' bacteria, that was first isolated from the variety "Tracy I" ( ATCC 10716) in 1945. These peptides disrupt Gram-positive bacteria by interfering with cell wall and peptidoglycan synthesis. Bacitracin is primarily used as a topical preparation, as it can cause kidney damage when used internally. It is generally safe when used topically, but in rare cases may cause hypersensitivity, allergic or anaphylactic reactions, especially in patient allergic to neomycin. Medical uses Bacitracin is used in human medicine as a polypeptide antibiotic and is "approved by the U.S. Food and Drug Administration (FDA) for use in chickens and turkeys," though use in animals contributes to antibiotic resistance. As bacitracin zinc salt, in combination with other topical antibiotics (usually polymyxin B and neomycin) as an ointment ("triple antibiotic ointment," with the brand n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the common cold or influenza; drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against microbes, but in the usual medical usage, antibiotics (such as penicillin) are those produced naturally (by one microorganism fighting another), whereas non-antibiotic antibacterials (such as sulfonamides and antiseptics) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Racemization
In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic (optically inactive) form. This creates a 1:1 molar ratio of enantiomers and is referred too as a racemic mixture (i.e. contain equal amount of (+) and (−) forms). Plus and minus forms are called Dextrorotation and levorotation. The D and L enantiomers are present in equal quantities, the resulting sample is described as a racemic mixture or a racemate. Racemization can proceed through a number of different mechanisms, and it has particular significance in pharmacology as different enantiomers may have different pharmaceutical effects. Stereochemistry Chiral molecules have two forms (at each point of asymmetry), which differ in their optical characteristics: The ''levorotatory form'' (the ''(−)-form'') will rotate counter-clockwise on the plane of polarization of a beam of light, whereas the ''dextrorotatory'' form (the ''(+)-form'') will rotate clockw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pipecolic Acid
Pipecolic acid (piperidine-2-carboxylic acid) is an organic compound with the formula HNC5H9CO2H. It is a carboxylic acid derivative of piperidine and, as such, an amino acid, although not one encoded genetically. Like many other α-amino acids, pipecolic acid is chiral, although the S-stereoisomer is more common. It is a colorless solid. Its biosynthesis starts from lysine. CRYM, a taxon-specific protein that also binds thyroid hormones, is involved in the pipecolic acid pathway. Medicine It accumulates in pipecolic acidemia. Pipecolic acid can be associated with some forms of epilepsy. Occurrence and reactions Like most amino acids, pipecolic acid is a chelating agent. One complex is Cu(HNC5H9CO2)2(H2O)2. Pipecolic acid was identified in the Murchison meteorite. It also occurs in the leaves of the genus ''Myroxylon'', a tree from South America. See also * Bupivacaine * Efrapeptin Efrapeptins are peptides produced by fungi in the genus ''Tolypocladium'' that have antifunga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketoacid
In organic chemistry, keto acids or ketoacids (also called oxo acids or oxoacids) are organic compounds that contain a carboxylic acid group () and a ketone group ().Franz Dietrich Klingler, Wolfgang Ebertz "Oxocarboxylic Acids" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. In several cases, the keto group is hydrated. The alpha-keto acids are especially important in biology as they are involved in the Krebs citric acid cycle and in glycolysis. Common types of keto acids include: *Alpha-keto acids, alpha-ketoacids, or 2-oxoacids have the keto group adjacent to the carboxylic acid. They often arise by oxidative deamination of amino acids, and reciprocally, they are precursors to the same. Alpha-keto acids possesses extensive chemistry as acylation agents. Furthermore, alpha-keto acids such as phenylpyruvic acid are endogenous sources for carbon monoxide (as a gasotransmitter) and pharmaceutical prodrug scaffold. Important representatives: ** p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
L-amino-acid Oxidase
In enzymology, an L-amino acid oxidase (LAAO) () is an enzyme that catalyzes the chemical reaction :an L-amino acid + H2O + O2 \rightleftharpoons a 2-oxo acid + NH3 + H2O2 The enzyme was first described in 1944 by A. Zeller and A. Maritz. Not only are LAAOs quite variable in terms of molecular mass, they also vary widely regarding stability. In a similar vein, this enzyme performs in a myriad of biological activities including apoptosis-induction, edema-induction, hemorrhaging, and inhibition or induction of platelet aggregation. As suggested by the name of the family, LAAOs are flavoenzymes which function to catalyze the stereospecific oxidative deamination of an L-amino acid. The three substrates of the enzymatic reaction are an L-amino acid, water, and oxygen, whereas the three products are the corresponding α-keto acid (2-oxo acid), ammonia, and hydrogen peroxide. One example of the enzyme in action occurs with the conversion L-alanine into pyruvic acid (2-oxopropanoic aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino-acid Racemase
In enzymology, an amino-acid racemase () is an enzyme that catalyzes the chemical reaction :an L-amino acid \rightleftharpoons a D-amino acid Hence, this enzyme has one substrate, L-amino acid, and one product, D-amino acid. This enzyme belongs to the family of isomerases, specifically those racemases and epimerases acting on amino acids and derivatives. The systematic name of this enzyme class is amino-acid racemase. This enzyme is also called L-amino acid racemase. This enzyme participates in 4 metabolic pathways: glycine, serine and threonine metabolism, cysteine metabolism, D-glutamine and D-glutamate metabolism, and D-arginine and D-ornithine metabolism. It employs one cofactor, pyridoxal phosphate. Structural studies As of late 2007, 5 structures A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
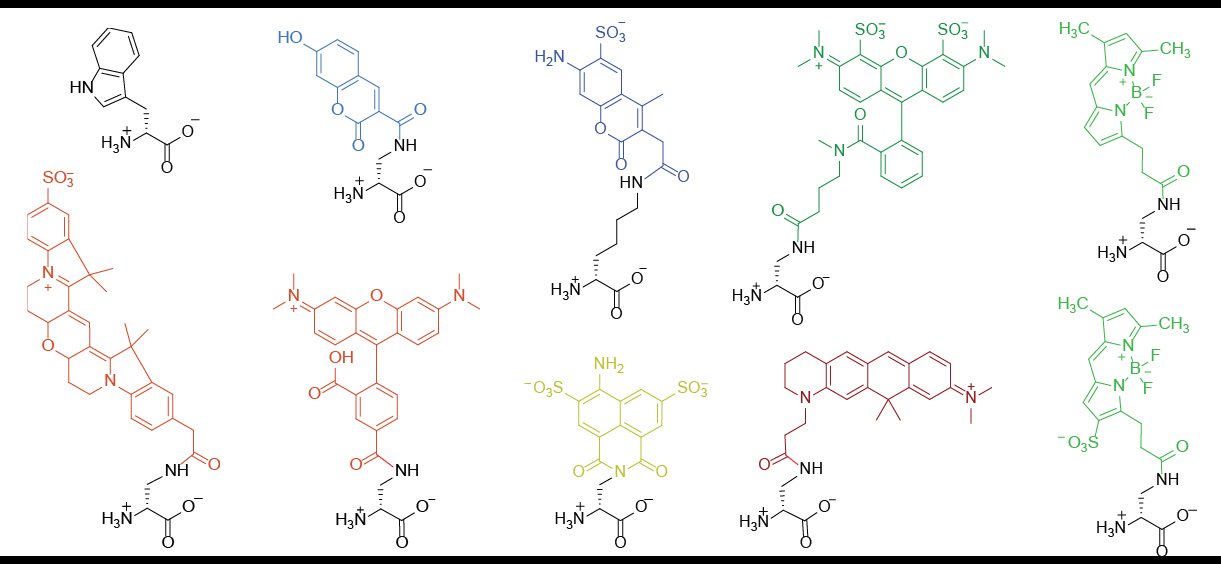
Fluorescent D-amino Acids
Fluorescent D-amino acids (FDAAs) are D-amino acid derivatives whose side-chain terminal is covalently coupled with a fluorophore molecule. FDAAs incorporate into the bacterial peptidoglycan (PG) in live bacteria, resulting in strong peripheral and septal PG labeling without affecting cell growth. They are featured with their ''in-situ'' incorporation mechanisms which enable time-course tracking of new PG formation. To date, FDAAs have been employed for studying the cell wall synthesis in various bacterial species (both Gram-positives and Gram-negatives) through different techniques, such as microscopy, mass spectrometry, flow cytometry. Structures and general properties FDAA consists of a D-amino acid and a fluorophore (coupled through the amino acid side chain.) The D-amino acid backbone is required for its incorporation into the bacterial peptidoglycan through the activity of DD-transpeptidases. Once being incorporated, one can use fluorescence-detection techniques to visua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Swiss-Prot
UniProt is a freely accessible database of protein sequence and functional information, many entries being derived from genome sequencing projects. It contains a large amount of information about the biological function of proteins derived from the research literature. It is maintained by the UniProt consortium, which consists of several European bioinformatics organisations and a foundation from Washington, DC, United States. The UniProt consortium The UniProt consortium comprises the European Bioinformatics Institute (EBI), the Swiss Institute of Bioinformatics (SIB), and the Protein Information Resource (PIR). EBI, located at the Wellcome Trust Genome Campus in Hinxton, UK, hosts a large resource of bioinformatics databases and services. SIB, located in Geneva, Switzerland, maintains the ExPASy (Expert Protein Analysis System) servers that are a central resource for proteomics tools and databases. PIR, hosted by the National Biomedical Research Foundation (NBRF) at the Geor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |