|
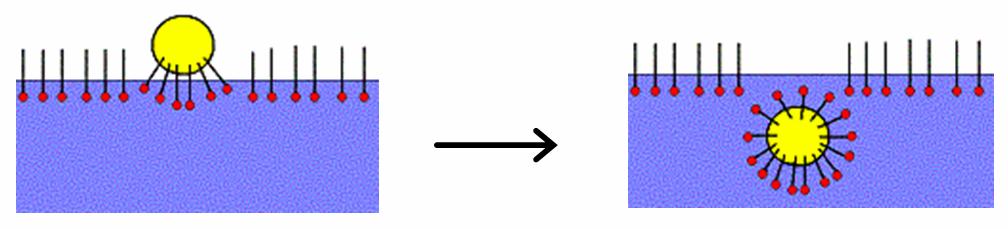
Critical Micelle Concentration
In colloidal and surface chemistry, the critical micelle concentration (CMC) is defined as the concentration of surfactants above which micelles form and all additional surfactants added to the system will form micelles. The CMC is an important characteristic of a surfactant. Before reaching the CMC, the surface tension changes strongly with the concentration of the surfactant. After reaching the CMC, the surface tension remains relatively constant or changes with a lower slope. The value of the CMC for a given dispersant in a given medium depends on temperature, pressure, and (sometimes strongly) on the presence and concentration of other surface active substances and electrolytes. Micelles only form above critical micelle temperature. For example, the value of CMC for sodium dodecyl sulfate in water (without other additives or salts) at 25 °C, atmospheric pressure, is 8x10−3 mol/L. Description Upon introducing surfactants (or any surface active materials) into a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work ( pharmacology), and how to collec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsion#Emulsifiers , emulsifiers, foaming agents, or dispersants. The word "surfactant" is a Blend word, blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to float on a water surface without becoming even partly submerged. At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to cohesion) than to the molecules in the air (due to adhesion). There are two primary mechanisms in play. One is an inward force on the surface molecules causing the liquid to contract. Second is a tangential force parallel to the surface of the liquid. This ''tangential'' force is generally referred to as the surface tension. The net effect is the liquid behaves as if its surface were covered with a stretched elastic membrane. But this analogy must not be taken too far as the tension in an elastic membrane is dependent on the amount of deformation of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are more soluble in hard water, because the polar sulfonate (of detergents) is less likely than the polar carboxylate (of soap) to bind to calcium and other ions found in hard water. Definitions The word ''detergent'' is derived from the Latin adjective ''detergens'', from the verb ''detergere'', meaning to wipe or polish off. Detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. However, conventionally, detergent is used to mean synthetic cleaning compounds as opposed to ''soap'' (a salt of the natural fatty acid), even though soap is also a detergent in the true sense. In domestic contexts, the term ''detergent'' refers to household cleaning products such as '' laundry detergent'' o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enhanced Oil Recovery
Enhanced oil recovery (abbreviated EOR), also called tertiary recovery, is the extraction of crude oil from an oil field that cannot be extracted otherwise. EOR can extract 30% to 60% or more of a reservoir's oil, compared to 20% to 40% using primary and secondary recovery. According to the US Department of Energy, carbon dioxide and water are injected along with one of three EOR techniques: thermal injection, gas injection, and chemical injection. More advanced, speculative EOR techniques are sometimes called quaternary recovery. Methods There are three primary techniques of EOR: gas injection, thermal injection, and chemical injection. Gas injection, which uses gases such as natural gas, nitrogen, or carbon dioxide (CO2), accounts for nearly 60 percent of EOR production in the United States. Thermal injection, which involves the introduction of heat, accounts for 40 percent of EOR production in the United States, with most of it occurring in California. Chemical injection, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubilization
Micellar solubilization (solubilization) is the process of incorporating the solubilizate (the component that undergoes solublization) into or onto micelles. Solublization may occur in a system consisting of a solvent, an association colloid (a colloid that forms micelles), and at least one other solubilizate. Usage of the term Solubilization is distinct from dissolution because the resulting fluid is a colloidal dispersion involving an association colloid. This suspension is distinct from a true solution, and the amount of the solubilizate in the micellar system can be different (often higher) than the regular solubility of the solubilizate in the solvent. In non-chemical literature and in everyday language, the term "solubilization" is sometimes used in a broader meaning as "to bring to a solution or (non- sedimenting) suspension" by any means, e.g., leaching by a reaction with an acid. Application Micellar solubilization is widely utilized, e.g. in laundry washing using d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Critical Micelle Concentration (CMC) Curve Attension Sigma 701
In colloidal and surface chemistry, the critical micelle concentration (CMC) is defined as the concentration of surfactants above which micelles form and all additional surfactants added to the system will form micelles. The CMC is an important characteristic of a surfactant. Before reaching the CMC, the surface tension changes strongly with the concentration of the surfactant. After reaching the CMC, the surface tension remains relatively constant or changes with a lower slope. The value of the CMC for a given dispersant in a given medium depends on temperature, pressure, and (sometimes strongly) on the presence and concentration of other surface active substances and electrolytes. Micelles only form above critical micelle temperature. For example, the value of CMC for sodium dodecyl sulfate in water (without other additives or salts) at 25 °C, atmospheric pressure, is 8x10−3 mol/L. Description Upon introducing surfactants (or any surface active materials) into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emulsion
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms ''colloid'' and ''emulsion'' are sometimes used interchangeably, ''emulsion'' should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid (the dispersed phase) is dispersed in the other (the continuous phase). Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working. Two liquids can form different types of emulsions. As an example, oil and water can form, first, an oil-in-water emulsion, in which the oil is the dispersed phase, and water is the continuous phase. Second, they can form a water-in-oil emulsion, in which water is the dispersed phase and oil is the continuous phase. Multiple emulsions are also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tensiometer (surface Tension)
In surface science, a tensiometer is a measuring instrument used to measure the surface tension () of liquids or surfaces. Tensiometers are used in research and development laboratories to determine the surface tension of liquids like coatings, lacquers or adhesives. A further application field of tensiometers is the monitoring of industrial production processes like parts cleaning or electroplating. Types Goniometer/Tensiometer Surface scientists commonly use an optical goniometer/tensiometer to measure the surface tension and interfacial tension of a liquid using the pendant or sessile drop methods. A drop is produced and captured using a CCD camera. The drop profile is subsequently extracted, and sophisticated software routines then fit the theoretical Young-Laplace equation to the experimental drop profile. The surface tension can then be calculated from the fitted parameters. Unlike other methods, this technique requires only a small amount of liquid making it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inflection Point
In differential calculus and differential geometry, an inflection point, point of inflection, flex, or inflection (British English: inflexion) is a point on a smooth plane curve at which the curvature changes sign. In particular, in the case of the graph of a function, it is a point where the function changes from being concave (concave downward) to convex (concave upward), or vice versa. For the graph of a function of differentiability class (''f'', its first derivative ''f, and its second derivative ''f'''', exist and are continuous), the condition ''f'' = 0'' can also be used to find an inflection point since a point of ''f'' = 0'' must be passed to change ''f'''' from a positive value (concave upward) to a negative value (concave downward) or vice versa as ''f'''' is continuous; an inflection point of the curve is where ''f'' = 0'' and changes its sign at the point (from positive to negative or from negative to positive). A point where the second derivative vanishes bu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_curve_Attension_Sigma_701.png)