|
Computational Thermodynamics
Computational thermodynamics is the use of computers to simulate thermodynamic problems specific to materials science, particularly used in the construction of phase diagrams. Several open and commercial programs exist to perform these operations. The concept of the technique is minimization of Gibbs free energy of the system; the success of this method is due not only to properly measuring thermodynamic properties, such as those in the list of thermodynamic properties, but also due to the extrapolation of the properties of metastable allotropes of the chemical elements. History The computational modeling of metal-based phase diagrams, which dates back to the beginning of the previous century mainly by Johannes van Laar and to the modeling of regular solutions, has evolved in more recent years to the CALPHAD (CALculation of PHAse Diagrams). This has been pioneered by American metallurgist Larry Kaufman since the 1970s. Current state Computational thermodynamics may be considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formulate a co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant typically need an additional 11% chromium. Because of its high tensile strength and low cost, steel is used in buildings, infrastructure, tools, ships, trains, cars, machines, electrical appliances, weapons, and rockets. Iron is the base metal of steel. Depending on the temperature, it can take two crystalline forms (allotropic forms): body-centred cubic and face-centred cubic. The interaction of the allotropes of iron with the alloying elements, primarily carbon, gives steel and cast iron their range of unique properties. In pure iron, the crystal structure has relatively little resistance to the iron atoms slipping past one another, and so pure iron is quite ductile, or soft and easily formed. In steel, small amounts of carbon, other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ETH Zurich
(colloquially) , former_name = eidgenössische polytechnische Schule , image = ETHZ.JPG , image_size = , established = , type = Public , budget = CHF 1.896 billion (2021) , rector = Günther Dissertori , president = Joël Mesot , academic_staff = 6,612 (including doctoral students, excluding 527 professors of all ranks, 34% female, 65% foreign nationals) (full-time equivalents 2021) , administrative_staff = 3,106 (40% female, 19% foreign nationals, full-time equivalents 2021) , students = 24,534 (headcount 2021, 33.3% female, 37% foreign nationals) , undergrad = 10,642 , postgrad = 8,299 , doctoral = 4,460 , other = 1,133 , address = Rämistrasse 101CH-8092 ZürichSwitzerland , city = Zürich , coor = , campus = Urban , language = German, English (Masters and upwards, sometimes Bachelor) , affiliations = CESAER, EUA, GlobalTech, IARU, IDEA League, UNITECH , website ethz.ch, colors = Black and White , logo = ETH Zürich Logo black.svg ETH Züric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UNIFAC
In statistical thermodynamics, the UNIFAC method ( UNIQUAC Functional-group Activity Coefficients)Aage Fredenslund, Russell L. Jones and John M. Prausnitz, "Group-Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures", ''AIChE Journal'', vol. 21 (1975), p. 1086 is a semi-empirical system for the prediction of non-electrolyte activity in non-ideal mixtures. UNIFAC uses the functional groups present on the molecules that make up the liquid mixture to calculate activity coefficients. By using interactions for each of the functional groups present on the molecules, as well as some binary interaction coefficients, the activity of each of the solutions can be calculated. This information can be used to obtain information on liquid equilibria, which is useful in many thermodynamic calculations, such as chemical reactor design, and distillation calculations. The UNIFAC model was first published in 1975 by Fredenslund, Jones and John Prausnitz, a group of chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
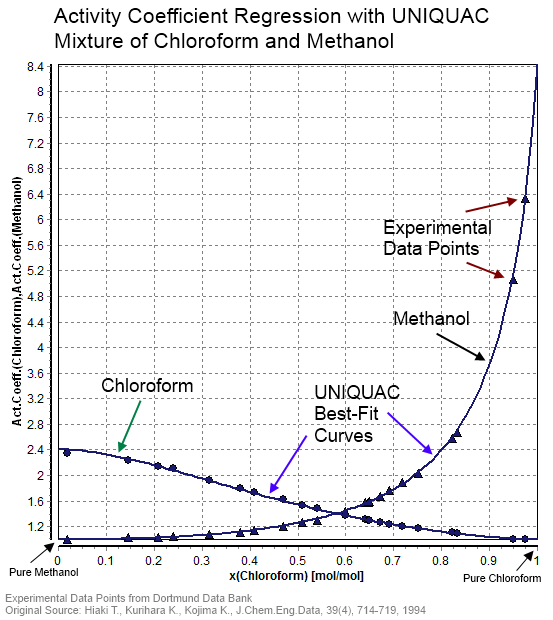
UNIQUAC
In statistical thermodynamics, UNIQUAC (a portmanteau of universal quasichemical) is an activity coefficient model used in description of phase equilibria. The model is a so-called lattice model and has been derived from a first order approximation of interacting molecule surfaces. The model is, however, not fully thermodynamically consistent due to its two- liquid mixture approach. In this approach the local concentration around one central molecule is assumed to be independent from the local composition around another type of molecule. The UNIQUAC model can be considered a second generation activity coefficient because its expression for the excess Gibbs energy consists of an entropy term in addition to an enthalpy term. Earlier activity coefficient models such as the Wilson equation and the non-random two-liquid model (NRTL model) only consist of enthalpy terms. Today the UNIQUAC model is frequently applied in the description of phase equilibria (i.e. liquid–solid, liquid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy Of Mixing
In thermodynamics, the enthalpy of mixing (also heat of mixing and excess enthalpy) is the enthalpy liberated or absorbed from a substance upon mixing. When a substance or compound is combined with any other substance or compound, the enthalpy of mixing is the consequence of the new interactions between the two substances or compounds. This enthalpy, if released exothermically, can in an extreme case cause an explosion. Enthalpy of mixing can often be ignored in calculations for mixtures where other heat terms exist, or in cases where the mixture is ideal. The sign convention is the same as for enthalpy of reaction: when the enthalpy of mixing is positive, mixing is endothermic, while negative enthalpy of mixing signifies exothermic mixing. In ideal mixtures, the enthalpy of mixing is null. In non-ideal mixtures, the thermodynamic activity of each component is different from its concentration by multiplying with the activity coefficient. One approximation for calculating the he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work (physics), work that may be performed by a closed system, thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as Chemical reaction, chemical reactions that may occur under these conditions. The Gibbs free energy change , measured in joules in International System of Units, SI) is the ''maximum'' amount of non-expansion work that can be extracted from a closed system (one that can exchange heat and work with its surroundings, but not matter) at fixed temperature and pressure. This maximum can be attained only in a completely reversible process (thermodynamics), reversible process. When a system transforms reversibly from an initial state to a final state under these conditions, the decrease in Gibbs free energy equals the work done by the system to its s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium. Overview Common components of a phase diagram are ''lines of equilibrium'' or ''phase boundaries'', which refer to lines that mark conditions under which multiple phases can coexist at equilibrium. Phase transitions occur along lines of equilibrium. Metastable phases are not shown in phase diagrams as, despite their common occurrence, they are not equilibrium phases. Triple points are points on phase diagrams where lines of equilibrium intersect. Triple points mark conditions at which three different phases can coexist. For example, the water phase diagram has a triple point corresponding to the single temperature and pressure at which solid, liquid, and gaseous water can coexist in a stabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vienna Ab-initio Simulation Package
The Vienna Ab initio Simulation Package, better known as VASP, is a package for performing ab initio quantum mechanical calculations using either Vanderbilt pseudopotentials, or the projector augmented wave method, and a plane wave basis set. The basic methodology is density functional theory (DFT), but the code also allows use of post-DFT corrections such as hybrid functionals mixing DFT and Hartree–Fock exchange (e.g. HSE, PBE0 or B3LYP), many-body perturbation theory (the GW method) and dynamical electronic correlations within the random phase approximation (RPA) and MP2. Originally, VASP was based on code written by Mike Payne (then at MIT), which was also the basis of CASTEP. It was then brought to the University of Vienna, Austria, in July 1989 by Jürgen Hafner. The main program was written by Jürgen Furthmüller, who joined the group at the Institut für Materialphysik in January 1993, and Georg Kresse. VASP is currently being developed by Georg Kresse; recent addit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, quantum field theory, quantum technology, and quantum information science. Classical physics, the collection of theories that existed before the advent of quantum mechanics, describes many aspects of nature at an ordinary (macroscopic) scale, but is not sufficient for describing them at small (atomic and subatomic) scales. Most theories in classical physics can be derived from quantum mechanics as an approximation valid at large (macroscopic) scale. Quantum mechanics differs from classical physics in that energy, momentum, angular momentum, and other quantities of a bound system are restricted to discrete values ( quantization); objects have characteristics of both particles and waves (wave–particle duality); and there are limits to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid State Physics
Solid-state physics is the study of rigid matter, or solids, through methods such as quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from their atomic-scale properties. Thus, solid-state physics forms a theoretical basis of materials science. It also has direct applications, for example in the technology of transistors and semiconductors. Background Solid materials are formed from densely packed atoms, which interact intensely. These interactions produce the mechanical (e.g. hardness and elasticity), thermal, electrical, magnetic and optical properties of solids. Depending on the material involved and the conditions in which it was formed, the atoms may be arranged in a regular, geometric pattern ( crystalline solids, which include metals and ordinary water ice) or irregularly (an amorphous solid such as common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |