|
Citrullination
Citrullination or deimination is the conversion of the amino acid arginine in a protein into the amino acid citrulline. Citrulline is not one of the 20 standard amino acids encoded by DNA in the genetic code. Instead, it is the result of a post-translational modification. Citrullination is distinct from the formation of the free amino acid citrulline as part of the urea cycle or as a byproduct of enzymes of the nitric oxide synthase family. Enzymes called arginine deiminases (ADIs) catalyze the deimination of free arginine, while protein arginine deiminases or peptidylarginine deiminases (PADs) replace the primary ketimine group (>C=NH) by a ketone group (>C=O). Arginine is positively charged at a neutral pH, whereas citrulline has no net charge. This increases the hydrophobicity of the protein, which can lead to changes in protein folding, affecting the structure and function. The immune system can attack citrullinated proteins, leading to autoimmune diseases such as rheumatoid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citrullination
Citrullination or deimination is the conversion of the amino acid arginine in a protein into the amino acid citrulline. Citrulline is not one of the 20 standard amino acids encoded by DNA in the genetic code. Instead, it is the result of a post-translational modification. Citrullination is distinct from the formation of the free amino acid citrulline as part of the urea cycle or as a byproduct of enzymes of the nitric oxide synthase family. Enzymes called arginine deiminases (ADIs) catalyze the deimination of free arginine, while protein arginine deiminases or peptidylarginine deiminases (PADs) replace the primary ketimine group (>C=NH) by a ketone group (>C=O). Arginine is positively charged at a neutral pH, whereas citrulline has no net charge. This increases the hydrophobicity of the protein, which can lead to changes in protein folding, affecting the structure and function. The immune system can attack citrullinated proteins, leading to autoimmune diseases such as rheumatoid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mutated Citrullinated Vimentin
Detection of autoantibodies against mutated citrullinated vimentin is part of rheumatoid arthritis (RA) diagnostics, especially in sera negative for rheumatoid factor (RF negative sera). Anti-MCV antibodies are a member of the ACPA family, a group of the so-called antibodies to citrullinated protein/peptide antigens. Rheumatoid arthritis is an autoimmune disorder. Detection of specific autoantibodies (antibodies directed against the body’s own tissue) such as rheumatoid factors and ACPAs may provide indication of the disease. In many cases, autoimmune diagnostics are crucial for diagnosing RA correctly and already in the disease’s early stages, when typical symptoms often are lacking but when medical therapy is most effective. Basics Citrullination is a modification of proteins where a nitrogen in the amino acid arginine is replaced with an oxygen, converting it into citrulline. The modified (citrullinated) protein may be identified by as foreign, provoking an autoimmune i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-translational Modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphate. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called glycosyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rheumatoid Factor
Rheumatoid factor (RF) is the autoantibody that was first found in rheumatoid arthritis. It is defined as an antibody against the Fc portion of IgG and different RFs can recognize different parts of the IgG-Fc. RF and IgG join to form immune complexes that contribute to the disease process. Rheumatoid factor can also be a cryoglobulin (antibody that precipitates on cooling of a blood sample); it can be either type 2 (monoclonal IgM to polyclonal IgG) or type 3 (polyclonal IgM to polyclonal IgG) cryoglobulin. Although ''predominantly'' encountered as IgM, rheumatoid factor can be of any isotype of immunoglobulins, i.e. IgA, IgG, IgM, IgE, IgD. Testing RF is tested by collecting blood in a plain tube (5 mL is often enough). The serum is tested for the presence of RF. There are different methods available, which include nephelometry, turbidimetry, agglutination of gamma globulin-coated latex particles or erythrocytes. RF is often evaluated in patients suspected of having any form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PAD3
Peptidyl arginine deiminase, type III, also known as PADI3, is a protein which in humans is encoded by the ''PADI3'' gene. This gene encodes a member of the peptidyl arginine deiminase family of enzymes, which catalyze the post-translational deimination of proteins by converting arginine residues into citrullines in the presence of calcium ions. The family members have distinct substrate specificities and tissue-specific expression patterns. The type III enzyme modulates hair structural proteins, such as filaggrin in the hair follicle and trichohyalin in the inner root sheath, during hair follicle formation. Together with the type I enzyme, this enzyme may also play a role in terminal differentiation of the epidermis. This gene exists in a cluster with four other paralogous genes. See also *Protein-arginine deiminase In enzymology, a protein-arginine deiminase () is an enzyme that catalyzes a form of post translational modification called arginine de-imination or citrulli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PAD2
Protein-arginine deiminase type-2 is an enzyme that in humans is encoded by the ''PADI2'' gene. This gene encodes a member of the peptidyl arginine deiminase family of enzymes, which catalyze the post-translational deimination of proteins by converting arginine residues into citrullines in the presence of calcium ions. The family members have distinct substrate specificities and tissue-specific expression patterns. The type II enzyme is the most widely expressed family member. Known substrates for this enzyme include myelin basic protein in the central nervous system and vimentin in skeletal muscle and macrophages. This enzyme is thought to play a role in the onset and progression of neurodegenerative human disorders, including Alzheimer disease and multiple sclerosis, and it has also been implicated in glaucoma pathogenesis. This gene exists in a cluster with four other paralogous Sequence homology is the biological homology between DNA, RNA, or protein sequences, de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PAD1
Peptidyl arginine deiminase, type I, also known as PADI1, is a protein which in humans is encoded by the ''PADI1'' gene. This gene encodes a member of the peptidyl arginine deiminase family of enzymes, which catalyze the post-translational deimination of proteins by converting arginine residues into citrullines in the presence of calcium ions. The family members have distinct substrate specificities and tissue-specific expression patterns. The type I enzyme is involved in the late stages of epidermal differentiation, where it deiminates filaggrin and keratin K1, which maintains hydration of the stratum corneum, and hence the cutaneous barrier function. This enzyme may also play a role in hair follicle formation. This gene exists in a cluster with four other paralogous Sequence homology is the biological homology between DNA, RNA, or protein sequences, defined in terms of shared ancestry in the evolutionary history of life. Two segments of DNA can have shared ancestry becau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
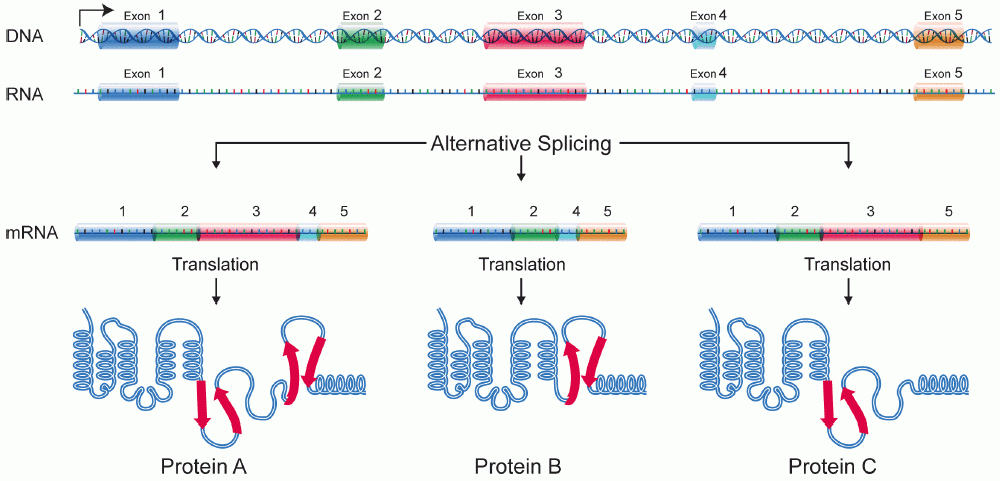
Isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene or gene family and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have unique functions. A set of protein isoforms may be formed from alternative splicings, variable promoter usage, or other post-transcriptional modifications of a single gene; post-translational modifications are generally not considered. (For that, see Proteoforms.) Through RNA splicing mechanisms, mRNA has the ability to select different protein-coding segments ( exons) of a gene, or even different parts of exons from RNA to form different mRNA sequences. Each unique sequence produces a specific form of a protein. The discovery of isoforms could explain the discrepancy between the small number of protein coding regions genes revealed by the human genome project and the large diversity of proteins seen in an organism: different ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food, energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. "Water" is also the name of the liquid state of H2O at standard temperature and pressure. A number of natural states of water exist. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Tertiary Structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may interact and bond in a number of ways. The interactions and bonds of side chains within a particular protein determine its tertiary structure. The protein tertiary structure is defined by its atomic coordinates. These coordinates may refer either to a protein domain or to the entire tertiary structure.Branden C. and Tooze J. "Introduction to Protein Structure" Garland Publishing, New York. 1990 and 1991. A number of tertiary structures may fold into a quaternary structure.Kyte, J. "Structure in Protein Chemistry." Garland Publishing, New York. 1995. History The science of the tertiary structure of proteins has progressed from one of hypothesis to one of detailed definition. Although Emil Fischer had suggested proteins were made of polypept ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-3D-balls.png)
