|
Cerium(III) Iodide
Cerium(III) iodide (CeI3) is the compound formed by cerium(III) cations and iodide An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine de ... anions. Preparation Cerium metal reacts with iodine when heated to form cerium(III) iodide: : It is also formed when cerium reacts with mercury(II) iodide at high temperatures: : Structure Cerium(III) iodide adopts the plutonium(III) bromide crystal structure. It contains 8-coordinate bicapped trigonal prismatic Ce3+ ions. Uses Cerium(III) iodide is used as a pharmaceutical intermediate and as a starting material for organocerium compounds. References {{Lanthanide halides Cerium(III) compounds Iodides Lanthanide halides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |
Inorganic Chemistry
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture. Key concepts Many inorganic compounds are ionic compounds, consisting of cations and anions joined by ionic bonding. Examples of salts (which are ionic compounds) are magnesium chloride MgCl2, which consists of magnesium cations Mg2+ and chloride anions Cl−; or sodium oxide Na2O, which consists of sodium cations Na+ and oxide anions O2−. In any salt, the proportions of the ions are such that the electric charges cancel out, so that the bulk compound is e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicapped Trigonal Prismatic
In chemistry, the bicapped trigonal prismatic molecular geometry describes the shape of compounds where eight atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a biaugmented triangular prism. This shape has C2v symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the square antiprism and the dodecahedron.Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford Science Publications It is very similar to the square antiprismatic molecular geometry, and there is some dispute over the specific geometry exhibited by certain molecules. One example of the bicapped trigonal prismatic molecular geometry is the ion An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven .... References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plutonium(III) Bromide
Plutonium(III) bromide is an inorganic salt of bromine and plutonium with the formula PuBr3. This radioactive green solid has few uses, however its crystal structure is often used as a structural archetype in crystallography. Crystal structure The PuBr3 crystal structure was first published in 1948 by William Houlder Zachariasen. The compound forms orthorhombic crystals, a type of square antiprism, within which the Pu atoms adopt an 8-coordinate bicapped trigonal prismatic arrangement. Its Pearson symbol is oS16 with the corresponding space group No. 63 (in International Union of Crystallography classification) or Cmcm (in Hermann–Mauguin notation In geometry, Hermann–Mauguin notation is used to represent the symmetry elements in point groups, plane groups and space groups. It is named after the German crystallographer Carl Hermann (who introduced it in 1928) and the French mineralogis ...). The majority of trivalent chloride and bromide salts of lanthanide and acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 oxidation state characteristic of the series, it also has a stable +4 state that does not oxidize water. It is also considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure. Despite always occurring in combination with the other rare-earth elements in minerals such as those of the monazite and bastnäsite groups, cerium is easy to extract from its ores, as it can be distinguished among the lanthanides by its unique ability to be oxidized to the +4 state in aqueous solution. It is the most common of the lanthanides, followed by neodymium, lanthanum, and praseodymium. It is the 25th-most abundant element, making up 66 ppm of the Ear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is not. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(II) Iodide
Mercury(II) iodide is a chemical compound with the molecular formula Hg I2. It is typically produced synthetically but can also be found in nature as the extremely rare mineral coccinite. Unlike the related mercury(II) chloride it is hardly soluble in water (<100 ppm). Production Mercury(II) iodide is produced by adding an aqueous solution of to an aqueous solution of with stirring; the precipitate is filtered off, washed and dried at 70 °C. : HgCl2 + 2 KI → HgI2 + 2 KClProperties Mercury(II) iodide displays |
Plutonium(III) Bromide
Plutonium(III) bromide is an inorganic salt of bromine and plutonium with the formula PuBr3. This radioactive green solid has few uses, however its crystal structure is often used as a structural archetype in crystallography. Crystal structure The PuBr3 crystal structure was first published in 1948 by William Houlder Zachariasen. The compound forms orthorhombic crystals, a type of square antiprism, within which the Pu atoms adopt an 8-coordinate bicapped trigonal prismatic arrangement. Its Pearson symbol is oS16 with the corresponding space group No. 63 (in International Union of Crystallography classification) or Cmcm (in Hermann–Mauguin notation In geometry, Hermann–Mauguin notation is used to represent the symmetry elements in point groups, plane groups and space groups. It is named after the German crystallographer Carl Hermann (who introduced it in 1928) and the French mineralogis ...). The majority of trivalent chloride and bromide salts of lanthanide and acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organocerium Chemistry
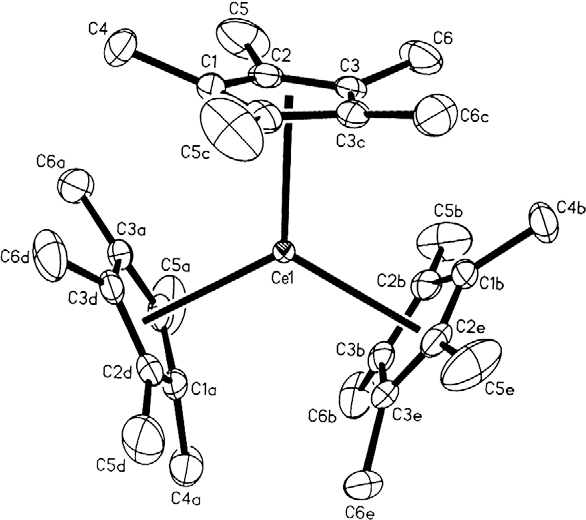
Organocerium chemistry is the science exploring the properties, structure, and reactivity of organocerium compounds, chemical compounds that contain one or more chemical bond between carbon and cerium. These compounds comprise a subset of the organolanthanides. In general, organocerium compounds are not isolable, and are rather studied in solution via their reactions with other species. There are notable exceptions, such as the Cp*3Ce(III) complex shown at right, but they are relatively rare. Complexes involving cerium of various oxidation states are known: though lanthanides are most stable in the +3 state, complexes of cerium(IV) have been reported. These latter compounds have found less widespread use due to their oxidizing nature, and the majority of literature regarding organometallic cerium complexes involves the +3 oxidation state. In particular, organocerium compounds have been developed extensively as non- basic carbon nucleophiles in organic synthesis. Because cerium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerium(III) Compounds
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 oxidation state characteristic of the series, it also has a stable +4 state that does not oxidize water. It is also considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure. Despite always occurring in combination with the other rare-earth elements in minerals such as those of the monazite and bastnäsite groups, cerium is easy to extract from its ores, as it can be distinguished among the lanthanides by its unique ability to be oxidized to the +4 state in aqueous solution. It is the most common of the lanthanides, followed by neodymium, lanthanum, and praseodymium. It is the 25th-most abundant element, making up 66 ppm of the E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodides
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is not. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


_oxide.jpg)