|
Caspase-1
Caspase-1/Interleukin-1 converting enzyme (ICE) is an evolutionarily conserved enzyme that proteolytically cleaves other proteins, such as the precursors of the inflammatory cytokines interleukin 1β and interleukin 18 as well as the pyroptosis inducer Gasdermin D, into active mature peptides. It plays a central role in cell immunity as an inflammatory response initiator. Once activated through formation of an inflammasome complex, it initiates a proinflammatory response through the cleavage and thus activation of the two inflammatory cytokines, interleukin 1β (IL-1β) and interleukin 18 (IL-18) as well as pyroptosis, a programmed lytic cell death pathway, through cleavage of Gasdermin D. The two inflammatory cytokines activated by Caspase-1 are excreted from the cell to further induce the inflammatory response in neighboring cells. Cellular expression Caspase-1 is evolutionarily conserved in many eukaryotes of the Kingdom Animalia. Due to its role in the inflammatory immune ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase-1 Zymogen
Caspase-1/Interleukin-1 converting enzyme (ICE) is an evolutionarily conserved enzyme that proteolytically cleaves other proteins, such as the precursors of the inflammatory cytokines interleukin 1β and interleukin 18 as well as the pyroptosis inducer Gasdermin D, into active mature peptides. It plays a central role in cell immunity as an inflammatory response initiator. Once activated through formation of an inflammasome complex, it initiates a proinflammatory response through the cleavage and thus activation of the two inflammatory cytokines, interleukin 1β (IL-1β) and interleukin 18 (IL-18) as well as pyroptosis, a programmed lytic cell death pathway, through cleavage of Gasdermin D. The two inflammatory cytokines activated by Caspase-1 are excreted from the cell to further induce the inflammatory response in neighboring cells. Cellular expression Caspase-1 is evolutionarily conserved in many eukaryotes of the Kingdom Animalia. Due to its role in the inflammatory immun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gasdermin D
Gasdermin D (GSDMD) is a protein that in humans is encoded by the ''GSDMD'' gene on chromosome 8. It belongs to the gasdermin family which is conserved among vertebrates and comprises six members in humans, GSDMA, GSDMB, GSDMC, GSDMD, GSDME (DFNA5) and DFNB59 (Pejvakin). Members of the gasdermin family are expressed in a variety of cell types including epithelial cells and immune cells. GSDMA, GSDMB, GSDMC, GSDMD and GSDME have been suggested to act as tumour suppressors. Structure The structure of full-length GSDMD consists of two domains, the 31 kDa N-terminal (GSDMD-N) and 22 kDa C-terminal (GSDMD-C) domains, separated by a linker region. GSDMD-C can be divided into four subdomains and is composed of 10 α-helices and two β-strands, forming a compact globular fold. The linker helix contacts the two helix-repeats which consist of four-helix bundles. The middle domain comprises an antiparallel β-strand and a short α-helix. The first flexible loop of GSDMD-C, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
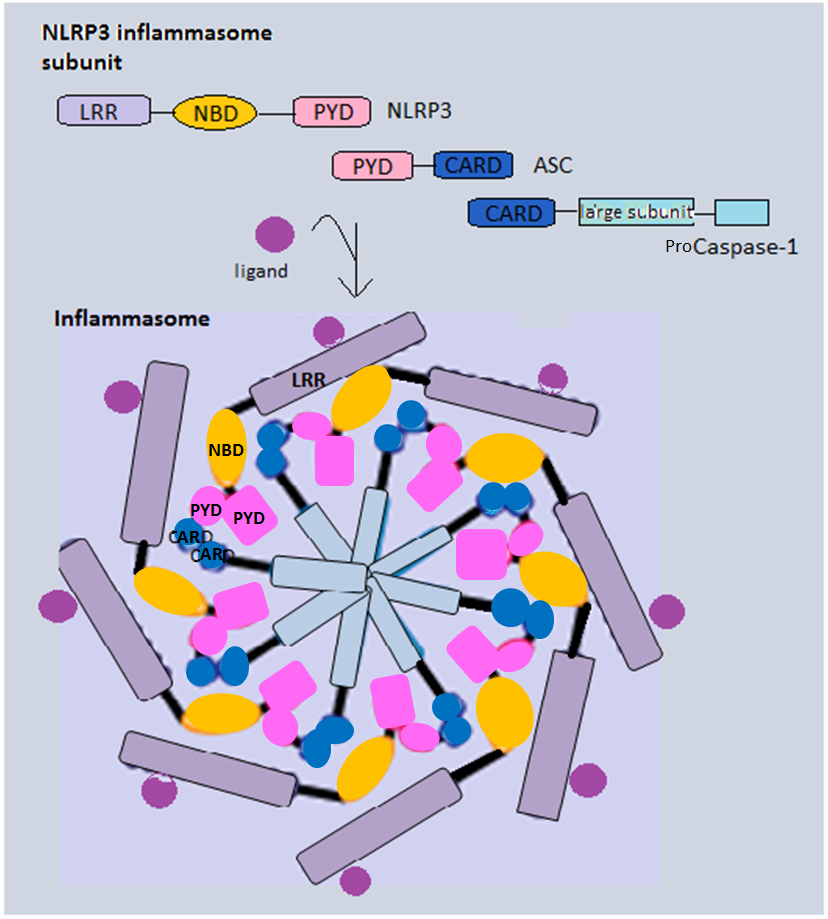
Inflammasome Vector
Inflammasomes are cytosolic multiprotein oligomers of the innate immune system responsible for the activation of inflammatory responses. Activation and assembly of the inflammasome promotes proteolytic cleavage, maturation and secretion of pro-inflammatory cytokines interleukin 1β (IL-1β) and interleukin 18 (IL-18), as well as cleavage of Gasdermin-D. The N-terminal fragment resulting from this cleavage induces a pro-inflammatory form of programmed cell death distinct from apoptosis, referred to as pyroptosis, and is responsible for secretion of the mature cytokines, presumably through the formation of pores in the plasma membrane. Inflammasome activation is initiated by different kinds of cytosolic pattern recognition receptors (PRRs) that respond to either microbe-derived pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) generated by the host cell. Pattern recognition receptors involved in inflammasomes comprise NLRs (nucle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inflammasome
Inflammasomes are cytosolic multiprotein oligomers of the innate immune system responsible for the activation of inflammatory responses. Activation and assembly of the inflammasome promotes proteolytic cleavage, maturation and secretion of pro-inflammatory cytokines interleukin 1β (IL-1β) and interleukin 18 (IL-18), as well as cleavage of Gasdermin-D. The N-terminal fragment resulting from this cleavage induces a pro-inflammatory form of programmed cell death distinct from apoptosis, referred to as pyroptosis, and is responsible for secretion of the mature cytokines, presumably through the formation of pores in the plasma membrane. Inflammasome activation is initiated by different kinds of cytosolic pattern recognition receptors (PRRs) that respond to either microbe-derived pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) generated by the host cell. Pattern recognition receptors involved in inflammasomes comprise NLRs (nucleoti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyroptosis
Pyroptosis is a highly inflammatory form of Lysis, lytic programmed cell death that occurs most frequently upon infection with intracellular pathogens and is likely to form part of the antimicrobial response. This process promotes the rapid clearance of various bacterial, viral, fungal and protozoan infections by removing intracellular replication niches and enhancing the host's defensive responses. Pyroptosis can take place in immune cells and is also reported to occur in keratinocytes and some epithelial cells. The process is initiated by formation of a large supramolecular complex termed the inflammasome (also known as a pyroptosome) upon intracellular danger signals. The inflammasome activates a different set of caspases as compared to apoptosis, for example, caspase-1/4/5 in humans and caspase-11 in mice. These caspases contribute to the maturation and activation of several proinflammatory cytokines and pore-forming protein GSDMD, gasdermins. Formation of pores causes cell membr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Belnacasan
Belnacasan (VX-765) is a drug developed by Vertex Pharmaceuticals which acts as a potent and selective inhibitor of the enzyme caspase 1. This enzyme is involved in inflammation and cell death, and consequently blocking its action may be useful for various medical applications, including treatment of epilepsy, arthritis, aiding recovery from heart attack and slowing the progression of Alzheimer's disease. Belnacasan is an orally active prodrug, being converted in the body to the active drug VRT-043198 (''O''-desethyl-belnacasan). However while belnacasan has proved well tolerated in human clinical trial Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, diet ...s, it has not shown sufficient efficacy to be approved for use for any of the applications suggested to date, though research cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NOD-like Receptor
The nucleotide-binding oligomerization domain-like receptors, or NOD-like receptors (NLRs) (also known as nucleotide-binding leucine-rich repeat receptors), are intracellular sensors of pathogen-associated molecular patterns (PAMPs) that enter the cell via phagocytosis or pores, and damage-associated molecular patterns (DAMPs) that are associated with cell stress. They are types of pattern recognition receptors (PRRs), and play key roles in the regulation of innate immune response. NLRs can cooperate with toll-like receptors (TLRs) and regulate inflammatory and apoptotic response. They are found in lymphocytes, macrophages, dendritic cells and also in non-immune cells, for example in epithelium. NLRs are highly conserved through evolution. Their homologs have been discovered in many different animal species (APAF1) and also in the plant kingdom ( disease-resistance R protein). Structure NLRs contain 3 domains – central NACHT (NOD or NBD – nucleotide-binding domain) domai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NLRC4
NLR family CARD domain-containing protein 4 is a protein that in humans is encoded by the ''NLRC4'' gene. Structure The NLRC4 protein is highly conserved across mammalian species. It bears homology to the ''C. elegans'' Ced4 protein. It contains an N-terminal CARD domain, a central nucleotide binding/ NACHT domain, and a C-terminal leucine rich repeat ( LRR) domain. It belongs to a family of NLR proteins that includes the transcriptional co-activator CIITA and the canonical inflammasome protein NLRP3. A truncated murine NLRC4 was the first member of this family whose crystal structure was solved. Function NLRC4 is best associated with triggering formation of the inflammasome. Unlike NLRP3, certain inflammasome-dependent functions of NLRC4 may be carried out independently of the inflammasome scaffold ASC. Human Ced4 homologs include APAF1, NOD1 (CARD4), and NOD2 (CARD15). These proteins have at least 1 N-terminal CARD domain followed by a centrally located nucleotide-bindi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CARD Domain
Caspase recruitment domains, or caspase activation and recruitment domains (CARDs), are interaction motifs found in a wide array of proteins, typically those involved in processes relating to inflammation and apoptosis. These domains mediate the formation of larger protein complexes via direct interactions between individual CARDs. CARD domains are found on a strikingly wide range of proteins, including helicases, kinases, mitochondrial proteins, caspases, and other cytoplasmic factors. Basic features CARD domains are a subclass of protein motif known as the death fold, which features an arrangement of six to seven antiparallel alpha helices with a hydrophobic core and an outer face composed of charged residues. Other motifs in this class include the pyrin domain (PYD), death domain (DD), and death effector domain (DED), all of which also function primarily in regulation of apoptosis and inflammatory responses. In apoptosis CARD domains were originally characterized based on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin 18
Interleukin-18 (IL-18), also known as interferon-gamma inducing factor) is a protein which in humans is encoded by the ''IL18'' gene. The protein encoded by this gene is a proinflammatory cytokine. Many cell types, both hematopoietic cells and non-hematopoietic cells, have the potential to produce IL-18. It was first described in 1989 as a factor that induced interferon-γ (IFN-γ) production in mouse spleen cells. Originally, IL-18 production was recognized in Kupffer cells, liver-resident macrophages. However, IL-18 is constitutively expressed in non-hematopoietic cells, such as intestinal epithelial cells, keratinocytes, and endothelial cells. IL-18 can modulate both innate and adaptive immunity and its dysregulation can cause autoimmune or inflammatory diseases. Processing Cytokines usually contain the signal peptide which is necessary for their extracellular release. In this case, ''IL18'' gene, similar to other IL-1 family members, lacks this signal peptide. Furthermore, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zymogen
In biochemistry, a zymogen (), also called a proenzyme (), is an inactive precursor of an enzyme. A zymogen requires a biochemical change (such as a hydrolysis reaction revealing the active site, or changing the configuration to reveal the active site) for it to become an active enzyme. The biochemical change usually occurs in Golgi bodies, where a specific part of the precursor enzyme is cleaved in order to activate it. The inactivating piece which is cleaved off can be a peptide unit, or can be independently-folding domains comprising more than 100 residues. Although they limit the enzyme's ability, these N-terminal extensions of the enzyme or a “prosegment” often aid in the stabilization and folding of the enzyme they inhibit. The pancreas secretes zymogens partly to prevent the enzymes from digesting proteins in the cells in which they are synthesised. Enzymes like pepsin are created in the form of pepsinogen, an inactive zymogen. Pepsinogen is activated when chief ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relative molecular mass, the structure of which essentially comprises a small plurality of units derived, actually or conceptually, from molecules of lower relative molecular mass.'' The name is composed of Greek elements '' oligo-'', "a few" and '' -mer'', "parts". An adjective form is ''oligomeric''. The oligomer concept is contrasted to that of a polymer, which is usually understood to have a large number of units, possibly thousands or millions. However, there is no sharp distinction between these two concepts. One proposed criterion is whether the molecule's properties vary significantly with the removal of one or a few of the units. An oligomer with a specific number of units is referred to by the Greek prefix denoting that number, wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |