|
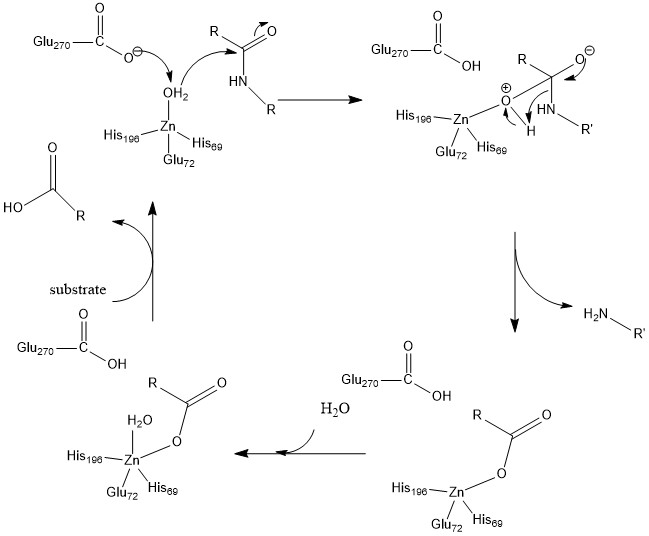
Carboxypeptidase
A carboxypeptidase ( EC number 3.4.16 - 3.4.18) is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation. At least two mechanisms have been discussed. Functions Initial studies on carboxypeptidases focused on pancreatic carboxypeptidases A1, A2, and B in the digestion of food. Most carboxypeptidases are not, however, involved in catabolism. Instead they help to mature proteins, for example Post-translational modification. They also regulate biological processes, such as the biosynthesis of neuroendocrine peptides such as insulin requires a carboxypeptidase. Carboxypeptidases also function in blood clotting, growth factor production, wound healing, reproduction, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxypeptidase A
Carboxypeptidase A usually refers to the pancreatic exopeptidase that hydrolyzes peptide bonds of C-terminal residues with aromatic or aliphatic side-chains. Most scientists in the field now refer to this enzyme as CPA1, and to a related pancreatic carboxypeptidase as CPA2. Types In addition, there are 4 other mammalian enzymes named CPA-3 through CPA-6, and none of these are expressed in the pancreas. Instead, these other CPA-like enzymes have diverse functions. * CPA3 (also known as mast-cell CPA) is involved in the digestion of proteins by mast cells. * CPA4 (previously known as CPA-3, but renumbered when mast-cell CPA was designated CPA-3) may be involved in tumor progression, but this enzyme has not been well studied. * CPA5 has not been well studied. * CPA6 is expressed in many tissues during mouse development, and in adult shows a more limited distribution in brain and several other tissues. CPA6 is present in the extracellular matrix where it is enzymatically ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxypeptidase A
Carboxypeptidase A usually refers to the pancreatic exopeptidase that hydrolyzes peptide bonds of C-terminal residues with aromatic or aliphatic side-chains. Most scientists in the field now refer to this enzyme as CPA1, and to a related pancreatic carboxypeptidase as CPA2. Types In addition, there are 4 other mammalian enzymes named CPA-3 through CPA-6, and none of these are expressed in the pancreas. Instead, these other CPA-like enzymes have diverse functions. * CPA3 (also known as mast-cell CPA) is involved in the digestion of proteins by mast cells. * CPA4 (previously known as CPA-3, but renumbered when mast-cell CPA was designated CPA-3) may be involved in tumor progression, but this enzyme has not been well studied. * CPA5 has not been well studied. * CPA6 is expressed in many tissues during mouse development, and in adult shows a more limited distribution in brain and several other tissues. CPA6 is present in the extracellular matrix where it is enzymatically ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxypeptidase B
Carboxypeptidase B (, ''protaminase'', ''pancreatic carboxypeptidase B'', ''tissue carboxypeptidase B'', ''peptidyl-L-lysine -arginineydrolase'') is a carboxypeptidase that preferentially acts upon basic amino acids, such as arginine and lysine. This serum enzyme is also responsible for rapidly metabolizing the C5a protein into C5a des-Arg, with one less amino acid. References External links * The MEROPS MEROPS is an online database for peptidases (also known as proteases, proteinases and proteolytic enzymes) and their inhibitors. The classification scheme for peptidases was published by Rawlings & Barrett in 1993, and that for protein inhibitor ... online database for peptidases and their inhibitorsM14.003* {{Portal, Biology EC 3.4.17 Metabolism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate Carboxypeptidase
Glutamate carboxypeptidase (, ''carboxypeptidase G'', ''carboxypeptidase G1'', ''carboxypeptidase G2'', ''glutamyl carboxypeptidase'', ''N-pteroyl-L-glutamate hydrolase'') is an enzyme. This enzyme catalyses the following chemical reaction : Release of C-terminal glutamate residues from a wide range of N-acylating moieties, including peptidyl, aminoacyl, benzoyl, benzyloxycarbonyl, folyl and pteroyl groups This zinc enzyme is produced by '' pseudomonads'', ''Flavobacterium'' sp. and ''Acinetobacter'' sp. See also * Glutamate carboxypeptidase II Glutamate carboxypeptidase II (GCPII), also known as N-acetyl-L-aspartyl-L-glutamate peptidase I (NAALADase I), NAAG peptidase, or prostate-specific membrane antigen (PSMA) is an enzyme that in humans is encoded by the ''FOLH1'' (folate hydrola ... References External links * {{Portal bar, Biology, border=no EC 3.4.17 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminopeptidase
Aminopeptidases are enzymes that catalyze the cleavage of amino acids from the amino terminus (N-terminus) of proteins or peptides (exopeptidases). They are widely distributed throughout the animal and plant kingdoms and are found in many subcellular organelles, in cytosol, and as membrane components. Aminopeptidases are used in essential cellular functions. Many, but not all, of these peptidases are zinc metalloenzymes. Some aminopeptidases are monomeric, and others are assemblies of relatively high mass (50 kDa) subunits. cDNA sequences are available for several aminopeptidases and a crystal structure of the open state of human endoplasmic reticulum Aminopeptidase 1 ERAP1 is presented here. Amino acid sequences determined directly or deduced from cDNAs indicate some amino acid sequence homologies in organisms as diverse as ''Escherichia coli'' and mammals, particularly in catalytically important residues or in residues involved in metal ion binding. One important aminopeptidas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reproduction
Reproduction (or procreation or breeding) is the biological process by which new individual organisms – "offspring" – are produced from their "parent" or parents. Reproduction is a fundamental feature of all known life; each individual organism exists as the result of reproduction. There are two forms of reproduction: asexual and sexual. In asexual reproduction, an organism can reproduce without the involvement of another organism. Asexual reproduction is not limited to single-celled organisms. The cloning of an organism is a form of asexual reproduction. By asexual reproduction, an organism creates a genetically similar or identical copy of itself. The evolution of sexual reproduction is a major puzzle for biologists. The two-fold cost of sexual reproduction is that only 50% of organisms reproduce and organisms only pass on 50% of their genes.John Maynard Smith ''The Evolution of Sex'' 1978. Sexual reproduction typically requires the sexual interaction of two specializ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chemistry)
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form Hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their applica .... A base was therefore a metal hydroxide such as Sodium hydroxide, NaOH or Calcium hydroxide, Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine protease ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the -arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid. History Arginine was first isolated in 1886 from yellow lupin seedlings by the German chemist Ernst Schulze and his assistant Ernst Steiger. He named it from the Greek ''árgyros'' (ἄργυρος) meaning "silver" due to the silver-white appearance of arginine nitrate crystals. In 1897, Schulze and Ernst Winterstein (1865–1949) determined the structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds, whether straight or branched, and which contain no rings of any type, are always aliphatic. Cyclic compounds can be aliphatic if they are not aromatic. Structure Aliphatic compounds can be saturated, joined by single bonds (alkanes), or unsaturated, with double bonds (alkenes) or triple bonds (alkynes). If other elements (heteroatoms) are bound to the carbon chain, the most common being oxygen, nitrogen, sulfur, and chlorine, it is no longer a hydrocarbon, and therefore no longer an aliphatic compound. The least complex aliphatic compound is methane (CH4). Properties Most aliphatic compounds are flammable, allowing the use of hydrocarbons as fuel, such as methane in Buns ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |