Carboxypeptidase A on:
[Wikipedia]
[Google]
[Amazon]
Carboxypeptidase A usually refers to the
 The active site can be characterized into two sub-sites denoted as S1’ and S1. The S1’ sub-site is the hydrophobic pocket of the enzyme, and Tyr-248 acts to ‘cap’ the hydrophobic pocket after substrate or inhibitor is bound (SITE). The hydrogen bonding from the hydroxyl group in Tyr-248 facilitates this conformation due to interaction with the terminal carboxylates of substrates that bind. Substantial movement is required for this enzyme and induced fit model explains how this interaction occurs.
A triad of residues interact to the C-terminal carboxylate through hydrogen bonding:
* Salt linkage with positively charged Arg-145
* Hydrogen bond from Tyr-248
* Hydrogen bond from the nitrogen of the Asn-144 amide
The active site can be characterized into two sub-sites denoted as S1’ and S1. The S1’ sub-site is the hydrophobic pocket of the enzyme, and Tyr-248 acts to ‘cap’ the hydrophobic pocket after substrate or inhibitor is bound (SITE). The hydrogen bonding from the hydroxyl group in Tyr-248 facilitates this conformation due to interaction with the terminal carboxylates of substrates that bind. Substantial movement is required for this enzyme and induced fit model explains how this interaction occurs.
A triad of residues interact to the C-terminal carboxylate through hydrogen bonding:
* Salt linkage with positively charged Arg-145
* Hydrogen bond from Tyr-248
* Hydrogen bond from the nitrogen of the Asn-144 amide
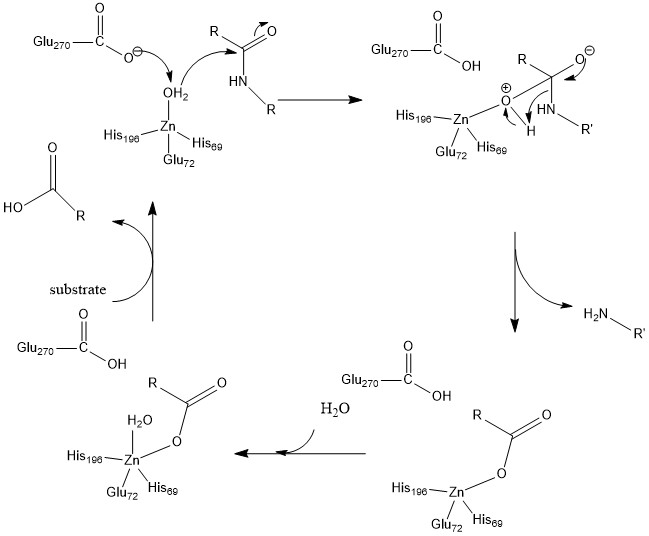 Glu-270 and Arg-127 play an important role in catalysis shown in Figure 2. Arg-127 acts to stabilize the carbonyl of the substrate that is bound to amino group of phenylalanine. Simultaneously, the water molecule coordinated to zinc is deprotonated by Glu-270 and interacts with the carbonyl stabilized by Arg-127. This creates an intermediate, shown in Figure 2, where the negatively charged oxygen is coordinated to zinc, and through unfavorable electrostatic interactions between Glu-270 and the ionized product facilitates the release of the product at the end of catalysis.
In recent computational studies, the mechanism of catalysis is similar but the difference in mechanism is that deprotonated water molecule binds to the carbon of the carbonyl, whereas Figure 2 shows the hydroxyl group stays coordinated to zinc. Then proteolysis occurs and the water molecule is then introduced back into the active site to coordinate to zinc.
Several studies have been conducted exploring the details of the bond between carboxypeptidase A and substrate and how this affects the rate of hydrolysis. In 1934, it was first discovered through kinetic experiments that, in order for substrate to bind, the peptide that is to be hydrolyzed must be adjacent to a terminal free hydroxyl group. Also, the rate of hydrolysis can be enhanced if the C-terminal residue is branched aliphatic or aromatic. However, if the substrate is a dipeptide with a free amino group, it undergoes hydrolysis slowly; this, however, can be avoided if the amino group is blocked by N-acylation.
It is quite clear that the structure of the enzyme, to be specific the active site, is very important in understanding the mechanism of reaction. For this reason, Rees and colleagues studied the enzyme-ligand complex to get a clear answer for the role of the zinc ion. These studies found that, in free enzyme, the zinc coordination number is five; the metal center is coordinated with two imidazole Nδ1 nitrogens, the two carboxylate oxygens of glutamate-72, and a water molecule to form a distorted tetrahedral. However, once ligand binds at the active site of carboxypeptidase A, this coordination number can vary from five to six. When bound to dipeptide glycyl-L-tyrosine, the amino nitrogen of the dipeptide and the carbonyl oxygen replaced the water ligand. This would yield a coordination number of six for the zinc in the carboxypeptidase A- dipeptide glycyl-L-tyrosine complex. Electron density maps gave evidence that the amino nitrogen occupies a second position near glutamate-270. The closeness of these two residues would result in a steric hindrance preventing the water ligand from coordinating with zinc. This would result in a coordination number of five. Data for both are substantial, indicating that both situations occur naturally.
There are two proposed mechanisms for the catalytic function of carboxypeptidase A. The first is a nucleophilic pathway involving a covalent acyl enzyme intermediate containing active site base Glu-270. Evidence for this anhydride intermediate is mixed; Suh and colleagues isolated what is assumed to by the acyl intermediate. However, confirmation of the acyl enzyme was done without trapping experiments, making the conclusions weak.
The second proposed mechanism is a promoted water pathway. This mechanism involves attack of a water molecule at the scissile peptide linkage of the substrate. This process is promoted by the zinc ion and assisted by residue Glu-270.
Glu-270 and Arg-127 play an important role in catalysis shown in Figure 2. Arg-127 acts to stabilize the carbonyl of the substrate that is bound to amino group of phenylalanine. Simultaneously, the water molecule coordinated to zinc is deprotonated by Glu-270 and interacts with the carbonyl stabilized by Arg-127. This creates an intermediate, shown in Figure 2, where the negatively charged oxygen is coordinated to zinc, and through unfavorable electrostatic interactions between Glu-270 and the ionized product facilitates the release of the product at the end of catalysis.
In recent computational studies, the mechanism of catalysis is similar but the difference in mechanism is that deprotonated water molecule binds to the carbon of the carbonyl, whereas Figure 2 shows the hydroxyl group stays coordinated to zinc. Then proteolysis occurs and the water molecule is then introduced back into the active site to coordinate to zinc.
Several studies have been conducted exploring the details of the bond between carboxypeptidase A and substrate and how this affects the rate of hydrolysis. In 1934, it was first discovered through kinetic experiments that, in order for substrate to bind, the peptide that is to be hydrolyzed must be adjacent to a terminal free hydroxyl group. Also, the rate of hydrolysis can be enhanced if the C-terminal residue is branched aliphatic or aromatic. However, if the substrate is a dipeptide with a free amino group, it undergoes hydrolysis slowly; this, however, can be avoided if the amino group is blocked by N-acylation.
It is quite clear that the structure of the enzyme, to be specific the active site, is very important in understanding the mechanism of reaction. For this reason, Rees and colleagues studied the enzyme-ligand complex to get a clear answer for the role of the zinc ion. These studies found that, in free enzyme, the zinc coordination number is five; the metal center is coordinated with two imidazole Nδ1 nitrogens, the two carboxylate oxygens of glutamate-72, and a water molecule to form a distorted tetrahedral. However, once ligand binds at the active site of carboxypeptidase A, this coordination number can vary from five to six. When bound to dipeptide glycyl-L-tyrosine, the amino nitrogen of the dipeptide and the carbonyl oxygen replaced the water ligand. This would yield a coordination number of six for the zinc in the carboxypeptidase A- dipeptide glycyl-L-tyrosine complex. Electron density maps gave evidence that the amino nitrogen occupies a second position near glutamate-270. The closeness of these two residues would result in a steric hindrance preventing the water ligand from coordinating with zinc. This would result in a coordination number of five. Data for both are substantial, indicating that both situations occur naturally.
There are two proposed mechanisms for the catalytic function of carboxypeptidase A. The first is a nucleophilic pathway involving a covalent acyl enzyme intermediate containing active site base Glu-270. Evidence for this anhydride intermediate is mixed; Suh and colleagues isolated what is assumed to by the acyl intermediate. However, confirmation of the acyl enzyme was done without trapping experiments, making the conclusions weak.
The second proposed mechanism is a promoted water pathway. This mechanism involves attack of a water molecule at the scissile peptide linkage of the substrate. This process is promoted by the zinc ion and assisted by residue Glu-270.
M14.001
* {{Portal, Biology Proteins EC 3.4.17 Metabolism Zinc enzymes
pancreatic
The pancreas is an Organ (anatomy), organ of the digestive system and endocrine system of vertebrates. In humans, it is located in the abdominal cavity, abdomen behind the stomach and functions as a gland. The pancreas is a mixed or heterocrine ...
exopeptidase
An exopeptidase is any peptidase that catalyzes the cleavage of the terminal (or the penultimate) peptide bond; the process releases a single amino acid, dipeptide or a tripeptide from the peptide chain. Depending on whether the amino acid is rel ...
that hydrolyzes
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
peptide bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein cha ...
s of C-terminal residues with aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
or aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, ...
side-chains. Most scientists in the field now refer to this enzyme as CPA1, and to a related pancreatic carboxypeptidase
A carboxypeptidase ( EC number 3.4.16 - 3.4.18) is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds ...
as CPA2.
Types
In addition, there are 4 other mammalian enzymes named CPA-3 through CPA-6, and none of these are expressed in the pancreas. Instead, these other CPA-like enzymes have diverse functions. * CPA3 (also known as mast-cell CPA) is involved in the digestion of proteins bymast cell
A mast cell (also known as a mastocyte or a labrocyte) is a resident cell of connective tissue that contains many granules rich in histamine and heparin. Specifically, it is a type of granulocyte derived from the myeloid stem cell that is a par ...
s.
* CPA4 (previously known as CPA-3, but renumbered when mast-cell CPA was designated CPA-3) may be involved in tumor
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
progression, but this enzyme has not been well studied.
* CPA5 has not been well studied.
* CPA6 is expressed in many tissues during mouse development, and in adult shows a more limited distribution in brain and several other tissues. CPA6 is present in the extracellular matrix where it is enzymatically active. A human mutation of CPA-6 has been linked to Duane's syndrome
Duane syndrome is a congenital rare type of strabismus most commonly characterized by the inability of the human eye, eye to move outward. The syndrome was first described by ophthalmologists Jakob Stilling (1887) and Siegmund Türk (1896), and s ...
(abnormal eye movement). Recently, mutations in CPA6 were found to be linked to epilepsy. CPA6 is also one of several enzymes which degrade enkephalins
An enkephalin is a pentapeptide involved in regulating nociception in the body. The enkephalins are termed endogenous ligands, as they are internally derived and bind to the body's opioid receptors. Discovered in 1975, two forms of enkephali ...
.
Function
CPA-1 and CPA-2 (and, it is presumed, all other CPAs) employ a zinc ion within the protein for hydrolysis of the peptide bond at the C-terminal end of an amino acid residue. Loss of the zinc leads to loss of activity, which can be replaced easily by zinc, and also by some otherdivalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of an ...
metals (cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, pr ...
, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
). Carboxypeptidase A is produced in the pancreas and is crucial to many processes in the human body to include digestion, post-translational modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosome ...
of proteins, blood clotting, and reproduction.
Applications
This vast scope of functionality for a single protein makes it the ideal model for research regarding other zinc proteases of unknown structure. Recent biomedical research on collagenase, enkephlinase, and angiotensin-converting enzyme used carboxypeptidase A for inhibitor synthesis and kinetic testing. For example, a drug that treats high blood pressure, Captopril, was designed based on a carboxypeptidase A inhibitor. Carboxypeptidase A and the target enzyme of Captopril, angiotensin-converting enzyme, have very similar structures, as they both contain a zinc ion within the active site. This allowed for a potent carboxypeptidase A inhibitor to be used to inhibit the enzyme and, thus, lower blood pressure through the renin-angiotensin-aldosterone system.Structure
Carboxypeptidase A (CPA) contains a zinc (Zn2+) metal center in a tetrahedral geometry with amino acid residues in close proximity around zinc to facilitate catalysis and binding. Out of the 307 amino acids bonded in a peptide chain, the following amino acid residues are important for catalysis and binding; Glu-270, Arg-71, Arg-127, Asn-144, Arg-145, and Tyr-248. Figure 1 illustrates the tetrahedral zinc complex active site with the important amino acid residues that surround the complex.Christianson, D., W., and Lipscomb, W., N. (1989) ''Carboxypeptidase A.'' American Chemical Society, Vol (22): 62-69. The zinc metal is a strong electrophilic Lewis acid catalyst which stabilizes a coordinated water molecule as well as stabilizes the negative intermediates that occur throughout the hydrolytic reaction. Stabilization of both the coordinated water molecule and negative intermediates are assisted by polar residues in the active site which are in close proximity to facilitate hydrogen bonding. The active site can be characterized into two sub-sites denoted as S1’ and S1. The S1’ sub-site is the hydrophobic pocket of the enzyme, and Tyr-248 acts to ‘cap’ the hydrophobic pocket after substrate or inhibitor is bound (SITE). The hydrogen bonding from the hydroxyl group in Tyr-248 facilitates this conformation due to interaction with the terminal carboxylates of substrates that bind. Substantial movement is required for this enzyme and induced fit model explains how this interaction occurs.
A triad of residues interact to the C-terminal carboxylate through hydrogen bonding:
* Salt linkage with positively charged Arg-145
* Hydrogen bond from Tyr-248
* Hydrogen bond from the nitrogen of the Asn-144 amide
The active site can be characterized into two sub-sites denoted as S1’ and S1. The S1’ sub-site is the hydrophobic pocket of the enzyme, and Tyr-248 acts to ‘cap’ the hydrophobic pocket after substrate or inhibitor is bound (SITE). The hydrogen bonding from the hydroxyl group in Tyr-248 facilitates this conformation due to interaction with the terminal carboxylates of substrates that bind. Substantial movement is required for this enzyme and induced fit model explains how this interaction occurs.
A triad of residues interact to the C-terminal carboxylate through hydrogen bonding:
* Salt linkage with positively charged Arg-145
* Hydrogen bond from Tyr-248
* Hydrogen bond from the nitrogen of the Asn-144 amide
Mechanism
Classified as a metalloexopeptidase, carboxypeptidase A consists of a single polypeptide chain bound to a zinc ion. This characteristic metal ion is located within the active site of the enzyme, along with five amino acid residues that are involved in substrate binding: Arg-71, Arg-127, Asn-144, Arg-145, Tyr-248, and Glu-270. X-ray crystallographic studies have revealed five subsites on the protein. These allosteric sites are involved in creating the ligand-enzyme specificity seen in most bioactive enzymes. One of these subsites induces a conformational change at Tyr-248 upon binding of a substrate molecule at the primary active site. The phenolic hydroxyl of tyrosine forms a hydrogen bond with the terminal carboxylate of the ligand. In addition, a second hydrogen bond is formed between the tyrosine and a peptide linkage of longer peptide substrates. These changes make the bond between the enzyme and ligand, whether it is substrate or inhibitor, much stronger. This property of carboxypeptidase A led to the first clause ofDaniel E. Koshland, Jr.
Daniel Edward Koshland Jr. (March 30, 1920July 23, 2007) was an American biochemist. He reorganized the study of biology at the University of California, Berkeley, and was the editor of the leading U.S. science journal, ''Science'', from 1985 ...
’s “induced fit” hypothesis.
The S1 sub-site is where catalysis occurs in CPA, and the zinc ion is coordinated by Glu-72, His-69, and His-196 enzyme residues. A plane exists that bisects the active-site groove where residues Glu-270 and Arg-127 are on opposite sides of the zinc-water coupled complex. The zinc is electron rich due to glutamine ligands coordinating the zinc because before substrate binds, Glu-72 coordinates bidentate but shifts to monodentate after substrate binds. As a result, the zinc metal is not able to deprotonate the coordinated water molecule to make a hydroxyl nucleophile.
 Glu-270 and Arg-127 play an important role in catalysis shown in Figure 2. Arg-127 acts to stabilize the carbonyl of the substrate that is bound to amino group of phenylalanine. Simultaneously, the water molecule coordinated to zinc is deprotonated by Glu-270 and interacts with the carbonyl stabilized by Arg-127. This creates an intermediate, shown in Figure 2, where the negatively charged oxygen is coordinated to zinc, and through unfavorable electrostatic interactions between Glu-270 and the ionized product facilitates the release of the product at the end of catalysis.
In recent computational studies, the mechanism of catalysis is similar but the difference in mechanism is that deprotonated water molecule binds to the carbon of the carbonyl, whereas Figure 2 shows the hydroxyl group stays coordinated to zinc. Then proteolysis occurs and the water molecule is then introduced back into the active site to coordinate to zinc.
Several studies have been conducted exploring the details of the bond between carboxypeptidase A and substrate and how this affects the rate of hydrolysis. In 1934, it was first discovered through kinetic experiments that, in order for substrate to bind, the peptide that is to be hydrolyzed must be adjacent to a terminal free hydroxyl group. Also, the rate of hydrolysis can be enhanced if the C-terminal residue is branched aliphatic or aromatic. However, if the substrate is a dipeptide with a free amino group, it undergoes hydrolysis slowly; this, however, can be avoided if the amino group is blocked by N-acylation.
It is quite clear that the structure of the enzyme, to be specific the active site, is very important in understanding the mechanism of reaction. For this reason, Rees and colleagues studied the enzyme-ligand complex to get a clear answer for the role of the zinc ion. These studies found that, in free enzyme, the zinc coordination number is five; the metal center is coordinated with two imidazole Nδ1 nitrogens, the two carboxylate oxygens of glutamate-72, and a water molecule to form a distorted tetrahedral. However, once ligand binds at the active site of carboxypeptidase A, this coordination number can vary from five to six. When bound to dipeptide glycyl-L-tyrosine, the amino nitrogen of the dipeptide and the carbonyl oxygen replaced the water ligand. This would yield a coordination number of six for the zinc in the carboxypeptidase A- dipeptide glycyl-L-tyrosine complex. Electron density maps gave evidence that the amino nitrogen occupies a second position near glutamate-270. The closeness of these two residues would result in a steric hindrance preventing the water ligand from coordinating with zinc. This would result in a coordination number of five. Data for both are substantial, indicating that both situations occur naturally.
There are two proposed mechanisms for the catalytic function of carboxypeptidase A. The first is a nucleophilic pathway involving a covalent acyl enzyme intermediate containing active site base Glu-270. Evidence for this anhydride intermediate is mixed; Suh and colleagues isolated what is assumed to by the acyl intermediate. However, confirmation of the acyl enzyme was done without trapping experiments, making the conclusions weak.
The second proposed mechanism is a promoted water pathway. This mechanism involves attack of a water molecule at the scissile peptide linkage of the substrate. This process is promoted by the zinc ion and assisted by residue Glu-270.
Glu-270 and Arg-127 play an important role in catalysis shown in Figure 2. Arg-127 acts to stabilize the carbonyl of the substrate that is bound to amino group of phenylalanine. Simultaneously, the water molecule coordinated to zinc is deprotonated by Glu-270 and interacts with the carbonyl stabilized by Arg-127. This creates an intermediate, shown in Figure 2, where the negatively charged oxygen is coordinated to zinc, and through unfavorable electrostatic interactions between Glu-270 and the ionized product facilitates the release of the product at the end of catalysis.
In recent computational studies, the mechanism of catalysis is similar but the difference in mechanism is that deprotonated water molecule binds to the carbon of the carbonyl, whereas Figure 2 shows the hydroxyl group stays coordinated to zinc. Then proteolysis occurs and the water molecule is then introduced back into the active site to coordinate to zinc.
Several studies have been conducted exploring the details of the bond between carboxypeptidase A and substrate and how this affects the rate of hydrolysis. In 1934, it was first discovered through kinetic experiments that, in order for substrate to bind, the peptide that is to be hydrolyzed must be adjacent to a terminal free hydroxyl group. Also, the rate of hydrolysis can be enhanced if the C-terminal residue is branched aliphatic or aromatic. However, if the substrate is a dipeptide with a free amino group, it undergoes hydrolysis slowly; this, however, can be avoided if the amino group is blocked by N-acylation.
It is quite clear that the structure of the enzyme, to be specific the active site, is very important in understanding the mechanism of reaction. For this reason, Rees and colleagues studied the enzyme-ligand complex to get a clear answer for the role of the zinc ion. These studies found that, in free enzyme, the zinc coordination number is five; the metal center is coordinated with two imidazole Nδ1 nitrogens, the two carboxylate oxygens of glutamate-72, and a water molecule to form a distorted tetrahedral. However, once ligand binds at the active site of carboxypeptidase A, this coordination number can vary from five to six. When bound to dipeptide glycyl-L-tyrosine, the amino nitrogen of the dipeptide and the carbonyl oxygen replaced the water ligand. This would yield a coordination number of six for the zinc in the carboxypeptidase A- dipeptide glycyl-L-tyrosine complex. Electron density maps gave evidence that the amino nitrogen occupies a second position near glutamate-270. The closeness of these two residues would result in a steric hindrance preventing the water ligand from coordinating with zinc. This would result in a coordination number of five. Data for both are substantial, indicating that both situations occur naturally.
There are two proposed mechanisms for the catalytic function of carboxypeptidase A. The first is a nucleophilic pathway involving a covalent acyl enzyme intermediate containing active site base Glu-270. Evidence for this anhydride intermediate is mixed; Suh and colleagues isolated what is assumed to by the acyl intermediate. However, confirmation of the acyl enzyme was done without trapping experiments, making the conclusions weak.
The second proposed mechanism is a promoted water pathway. This mechanism involves attack of a water molecule at the scissile peptide linkage of the substrate. This process is promoted by the zinc ion and assisted by residue Glu-270.
See also
*Carboxypeptidase A inhibitor
In molecular biology, the carboxypeptidase A inhibitor family is a family of proteins which is represented by the well-characterised metallocarboxypeptidase A inhibitor (MCPI) from potatoes, which belongs to the MEROPS inhibitor family I37, clan IE ...
* Carboxypeptidase B
Carboxypeptidase B (, ''protaminase'', ''pancreatic carboxypeptidase B'', ''tissue carboxypeptidase B'', ''peptidyl-L-lysine -arginineydrolase'') is a carboxypeptidase that preferentially acts upon basic amino acids, such as arginine and lysi ...
* Carboxypeptidase
A carboxypeptidase ( EC number 3.4.16 - 3.4.18) is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds ...
* Carboxypeptidase E
Carboxypeptidase E (CPE), also known as carboxypeptidase H (CPH) and enkephalin convertase, is an enzyme that in humans is encoded by the ''CPE'' gene. This enzyme catalyzes the release of C-terminal arginine or lysine residues from polypeptides ...
References
External links
* TheMEROPS
MEROPS is an online database for peptidases (also known as proteases, proteinases and proteolytic enzymes) and their inhibitors. The classification scheme for peptidases was published by Rawlings & Barrett in 1993, and that for protein inhibitor ...
online database for peptidases and their inhibitorsM14.001
* {{Portal, Biology Proteins EC 3.4.17 Metabolism Zinc enzymes