|
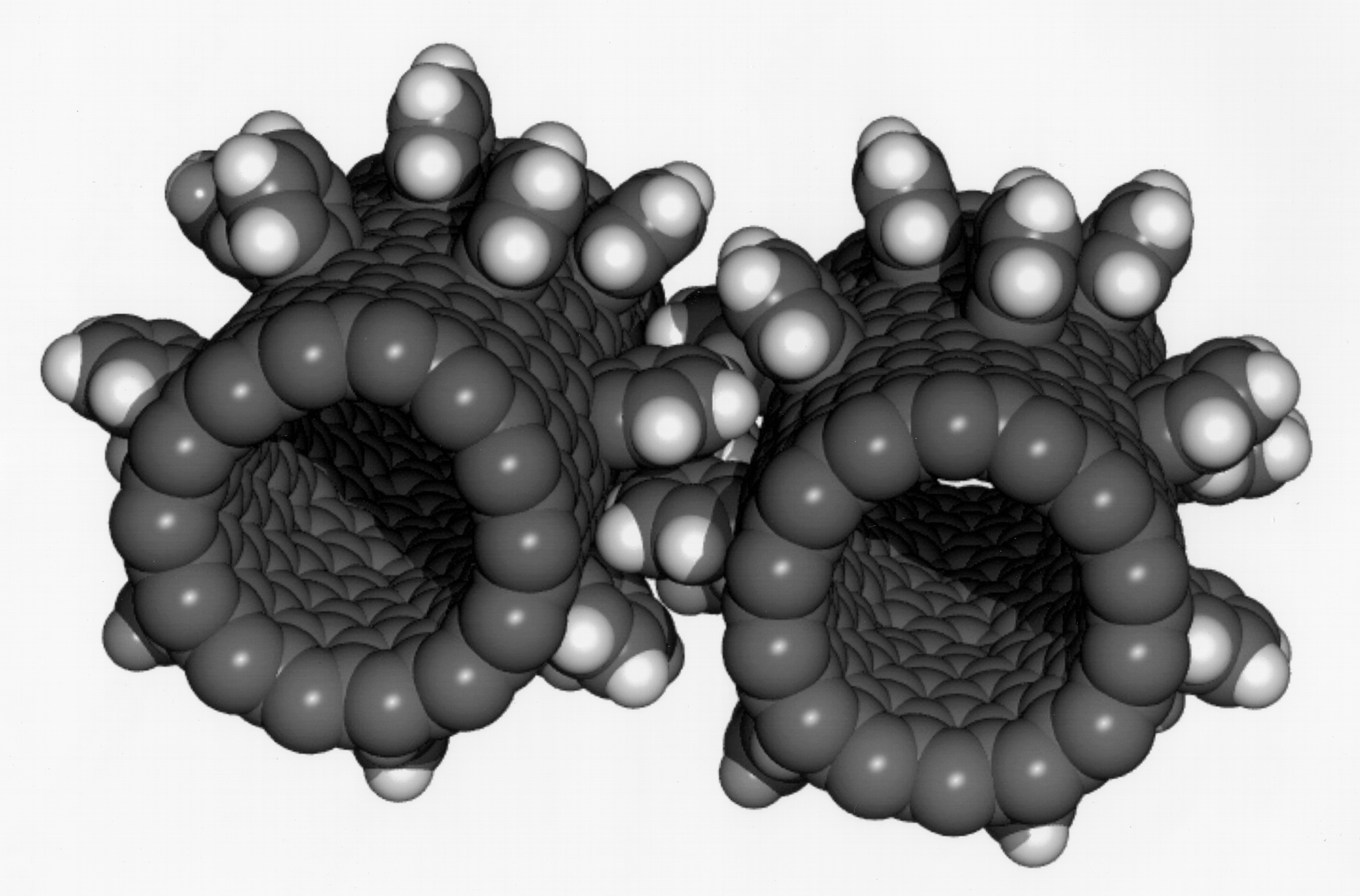
Carbide-derived Carbon
Carbide-derived carbon (CDC), also known as tunable nanoporous carbon, is the common term for carbon materials derived from carbide precursors, such as binary (e.g. SiC, TiC), or ternary carbides, also known as MAX phases (e.g., Ti2AlC, Ti3SiC2). CDCs have also been derived from polymer-derived ceramics such as Si-O-C or Ti-C, and carbonitrides, such as Si-N-C. CDCs can occur in various structures, ranging from amorphous to crystalline carbon, from sp2- to sp3-bonded, and from highly porous to fully dense. Among others, the following carbon structures have been derived from carbide precursors: micro- and mesoporous carbon, amorphous carbon, carbon nanotubes, onion-like carbon, nanocrystalline diamond, graphene, and graphite. Among carbon materials, microporous CDCs exhibit some of the highest reported specific surface areas (up to more than 3000 m2/g). By varying the type of the precursor and the CDC synthesis conditions, microporous and mesoporous structures with controllable avera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece. Interstitial / Metallic carbides The carbides of the group 4, 5 and 6 transition metals (with the exception of chromium) are often described as interstitial compounds. These carbides have metallic properties and are refractory. Some exhibit a range of stoichiometries, being a non-stoichiometric mixture of various carbides arising due to crystal defects. Some of them, including titanium carbide and tungsten carbide, are important industrially and are used to coat metals in cutting tools. The long-held view is that the carbon atoms fit into octahedral interstices in a close-packed metal lattice when the metal atom radius is greater than approximately 135 pm: *When the metal atoms are cubic close-packed, (ccp), then filling all of the octahedral interstices with carbon achieves 1:1 s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gleb Yushin
Gleb (Russian and be, Глеб) or Hlib ( uk, Гліб) is a Slavic male given name derived from the Old Norse name ''Guðleifr'', which means "heir of god." According to another version, the name Gleb comes from the name Olaf. It is popular in Russia due to an early martyr, Saint Gleb, who is venerated by Eastern Orthodox churches. Behind the Name It is also commonly used in Ukraine. Notable people with the name include: People * Gleb of Kiev (died 1171), Rus’ prince * Gleb Axelrod (1923–2003), Russian pianist * (1910–1976), Russi ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes Of Carbon
Carbon is capable of forming many allotropes (structurally different forms of the same element) due to its valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include carbon nano tube, nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA). Diamond Diamond is a well-known allotrope of carbon. The hardness, extremely high refractive index, and high dispersion of light make diamond useful for industrial applications and for jewelry. Diamond is the hardest known natural mineral. This makes it an excellent abrasive and makes it hold polish and luster extre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanoengineering
Nanoengineering is the practice of engineering on the nanoscale. It derives its name from the nanometre, a unit of measurement equalling one billionth of a meter. Nanoengineering is largely a synonym for nanotechnology, but emphasizes the engineering rather than the pure science aspects of the field. History * 4th Century Rome: The Lycurgus Cup was crafted using dichroic glass which is a product of nanoengineering * 6th-15th Centuries: Stained glass windows were created in European cathedrals which contained nanoparticles of gold chloride or other metal oxides or chlorides. These nanoparticles give the glass its vibrant colors. * 9th-17th Centuries: A sparkling layer on the outside of ceramics was used containing silver, copper, or other metallic nanoparticles. * 13th-18th Centuries: "Damascus" saber blades were crafted using techniques that resulted in nanotubes and cementite nanowires. * 1950: Victor La Mer and Robert Dinegar created a process that was used to create special ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanomaterials
* Nanomaterials describe, in principle, materials of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of nanoscale). Nanomaterials research takes a materials science-based approach to nanotechnology, leveraging advances in materials metrology and synthesis which have been developed in support of microfabrication research. Materials with structure at the nanoscale often have unique optical, electronic, thermo-physical or mechanical properties. Nanomaterials are slowly becoming commercialized and beginning to emerge as commodities. Definition In ISO/TS 80004, ''nanomaterial'' is defined as the "material with any external dimension in the nanoscale or having internal structure or surface structure in the nanoscale", with ''nanoscale'' defined as the "length range approximately from 1 nm to 100 nm". This includes both ''nano-objects'', which are discrete pieces of material, and ''nanostructured materials'', which have i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanotechnology
Nanotechnology, also shortened to nanotech, is the use of matter on an atomic, molecular, and supramolecular scale for industrial purposes. The earliest, widespread description of nanotechnology referred to the particular technological goal of precisely manipulating atoms and molecules for fabrication of macroscale products, also now referred to as molecular nanotechnology. A more generalized description of nanotechnology was subsequently established by the National Nanotechnology Initiative, which defined nanotechnology as the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (nm). This definition reflects the fact that quantum mechanical effects are important at this quantum-realm scale, and so the definition shifted from a particular technological goal to a research category inclusive of all types of research and technologies that deal with the special properties of matter which occur below the given size threshold. It is therefore commo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Economy
The hydrogen economy is using hydrogen to decarbonize economic sectors which are hard to electrify, essentially, the "hard-to-abate" sectors such as cement, steel, long-haul transport etc. In order to phase out fossil fuels and limit climate change, hydrogen can be created from water using renewable sources such as wind and solar, and its combustion only releases water vapor to the atmosphere. Hydrogen is an energetic fuel, frequently used as rocket fuel, but numerous technical challenges prevent the creation of a large-scale hydrogen economy. These include the difficulty of developing long-term storage, pipelines and engine equipment; a relative lack of off-the-shelf engine technology that can currently run safely on hydrogen; safety concerns regarding the high reactivity of hydrogen fuel with oxygen in ambient air; the expense of producing it by electrolysis; and a lack of efficient photochemical water splitting technology. Hydrogen can also react in a fuel cell, which effic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Storage
Hydrogen storage can be accomplished by several existing methods of holding hydrogen for later use. These include mechanical approaches such as using high pressures and low temperatures, or employing chemical compounds that release H2 upon demand. While large amounts of hydrogen are produced by various industries, it is mostly consumed at the site of production, notably for the synthesis of ammonia. For many years hydrogen has been stored as compressed gas or cryogenic liquid, and transported as such in cylinders, tubes, and cryogenic tanks for use in industry or as propellant in space programs. Interest in using hydrogen for on-board storage of energy in zero-emissions vehicles is motivating the development of new methods of storage, more adapted to this new application. The overarching challenge is the very low boiling point of H2: it boils around 20.268 K (−252.882 °C or −423.188 °F). Achieving such low temperatures requires expending significant energy. E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dangling Bond
In chemistry, a dangling bond is an unsatisfied valence on an immobilized atom. An atom with a dangling bond is also referred to as an immobilized free radical or an immobilized radical, a reference to its structural and chemical similarity to a free radical. When speaking of a dangling bond, one is generally referring to the state described above, containing one electron and thus leading to a neutrally charged atom. There are also dangling bond defects containing two or no electrons. These are negatively and positively charged respectively. Dangling bonds with two electrons have an energy close to the valence band of the material and those with none have an energy that is closer to the conduction band. Properties In order to gain enough electrons to fill their valence shells (see also octet rule), many atoms will form covalent bonds with other atoms. In the simplest case, that of a single bond, two atoms each contribute one unpaired electron, and the resulting pair of elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |