|
CTSL1
Cathepsin L1 is a protein that in humans is encoded by the ''CTSL1'' gene. The protein is a cysteine cathepsin, a lysosomal cysteine protease that plays a major role in intracellular protein catabolism. Function Cathepsin L1 is a member of the Peptidase C1 (cathepsin) MEROPS family, which plays an important role in diverse processes including normal lysosome mediated protein turnover, antigen and proprotein processing, and apoptosis. Its substrates include collagen and elastin, as well as alpha-1 protease inhibitor, a major controlling element of neutrophil elastase activity. The encoded protein has been implicated in several pathologic processes, including myofibril necrosis in myopathies and in myocardial ischemia, and in the renal tubular response to proteinuria. This protein, which is a member of the peptidase C1 family, is a dimer composed of disulfide-linked heavy and light chains, both produced from a single protein precursor. At least two transcript variants encoding the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystatin A
Cystatin-A is a protein that in humans is encoded by the ''CSTA'' gene. The cystatin superfamily encompasses proteins that contain multiple cystatin-like sequences. Some of the members are active cysteine protease inhibitors, while others have lost or perhaps never acquired this inhibitory activity. There are three inhibitory families in the superfamily, including the type 1 cystatins (stefins), type 2 cystatins, and kininogens. This gene encodes a stefin that functions as a cysteine protease inhibitor, forming tight complexes with papain and the cathepsins B, H, and L. The protein is one of the precursor proteins of cornified cell envelope in keratinocytes and plays a role in epidermal development and maintenance. Stefins have been proposed as prognostic and diagnostic tools for cancer. Interactions Cystatin A has been shown to interact with Cathepsin B and CTSL1. See also * Peptide Transporter Carbon Starvation (cstA) Family References Further reading * * * * * * * * * * * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide Bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. ''Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol (251& ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathepsins
Cathepsins (Ancient Greek ''kata-'' "down" and ''hepsein'' "boil"; abbreviated CTS) are proteases (enzymes that degrade proteins) found in all animals as well as other organisms. There are approximately a dozen members of this family, which are distinguished by their structure, catalytic mechanism, and which proteins they cleave. Most of the members become activated at the low pH found in lysosomes. Thus, the activity of this family lies almost entirely within those organelles. There are, however, exceptions such as cathepsin K, which works extracellularly after secretion by osteoclasts in bone resorption. Cathepsins have a vital role in mammalian cellular turnover. Classification * Cathepsin A (serine protease) * Cathepsin B (cysteine protease) * Cathepsin C (cysteine protease) * Cathepsin D (aspartyl protease) * Cathepsin E (aspartyl protease) * Cathepsin F (cysteine proteinase) * Cathepsin G (serine protease) * Cathepsin H (cysteine protease) * Cathepsin K (cysteine proteas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathepsin L2
Cathepsin L2, (, also known as cathepsin V or cathepsin U), is a protein encoded in humans by the CTSV gene. The protein is a human cysteine cathepsin, a lysosomal cysteine protease with endopeptidase activity. The protein is a member of the papain-like protease family (MEROPS family C1), a lysosomal cysteine protease with endopeptidase activity. It may play an important role in corneal physiology. This gene is expressed in colorectal and breast carcinomas but not in normal colon, mammary gland, or peritumoral tissues, suggesting a possible role for this gene in tumor processes. Clinical significance Cathepsin L2 may play a role in the pathogenesis of keratoconus. References External links * The MEROPS MEROPS is an online database for peptidases (also known as proteases, proteinases and proteolytic enzymes) and their inhibitors. The classification scheme for peptidases was published by Rawlings & Barrett in 1993, and that for protein inhibitor ... online database for p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
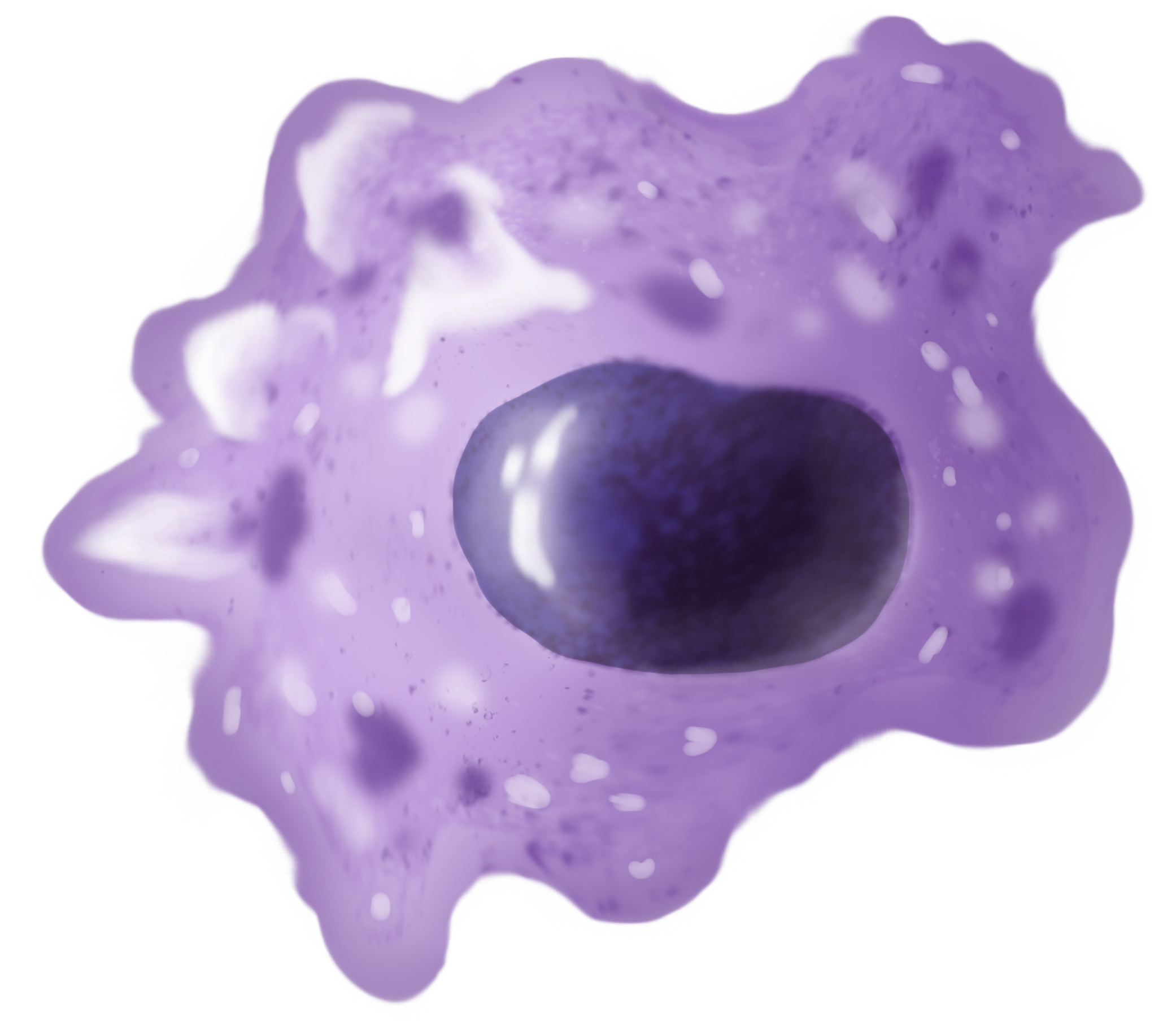
Macrophage
Macrophages (abbreviated as M φ, MΦ or MP) ( el, large eaters, from Greek ''μακρός'' (') = large, ''φαγεῖν'' (') = to eat) are a type of white blood cell of the immune system that engulfs and digests pathogens, such as cancer cells, microbes, cellular debris, and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. The process is called phagocytosis, which acts to defend the host against infection and injury. These large phagocytes are found in essentially all tissues, where they patrol for potential pathogens by amoeboid movement. They take various forms (with various names) throughout the body (e.g., histiocytes, Kupffer cells, alveolar macrophages, microglia, and others), but all are part of the mononuclear phagocyte system. Besides phagocytosis, they play a critical role in nonspecific defense (innate immunity) and also help initiate specific defense mechanisms (adaptive immunity) by recruiting other immune ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inflammation
Inflammation (from la, wikt:en:inflammatio#Latin, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or Irritation, irritants, and is a protective response involving immune cells, blood vessels, and molecular mediators. The function of inflammation is to eliminate the initial cause of cell injury, clear out necrotic cells and tissues damaged from the original insult and the inflammatory process, and initiate tissue repair. The five cardinal signs are heat, pain, redness, swelling, and Functio laesa, loss of function (Latin ''calor'', ''dolor'', ''rubor'', ''tumor'', and ''functio laesa''). Inflammation is a generic response, and therefore it is considered as a mechanism of innate immune system, innate immunity, as compared to adaptive immune system, adaptive immunity, which is specific for each pathogen. Too little inflammation could lead to progressive tissue destruction by the harmful stimulus (e.g. b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxychloroquine
Hydroxychloroquine, sold under the brand name Plaquenil among others, is a medication used to prevent and treat malaria in areas where malaria remains sensitive to chloroquine. Other uses include treatment of rheumatoid arthritis, lupus, and porphyria cutanea tarda. It is taken by mouth, often in the form of hydroxychloroquine sulfate. Common side effects may include vomiting, headache, changes in vision, and muscle weakness. Severe side effects may include allergic reactions, vision problems, and heart problems. Although all risk cannot be excluded, it remains a treatment for rheumatic disease during pregnancy. Hydroxychloroquine is in the antimalarial and 4-aminoquinoline families of medication. Hydroxychloroquine was approved for medical use in the United States in 1955. It is on the World Health Organization's List of Essential Medicines. In 2020, it was the 126th most commonly prescribed medication in the United States, with more than 4million prescriptions. Hydrox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endosome
Endosomes are a collection of intracellular sorting organelles in eukaryotic cells. They are parts of endocytic membrane transport pathway originating from the trans Golgi network. Molecules or ligands internalized from the plasma membrane can follow this pathway all the way to lysosomes for degradation or can be recycled back to the cell membrane in the endocytic cycle. Molecules are also transported to endosomes from the trans Golgi network and either continue to lysosomes or recycle back to the Golgi apparatus. Endosomes can be classified as early, sorting, or late depending on their stage post internalization. Endosomes represent a major sorting compartment of the endomembrane system in cells. Function Endosomes provide an environment for material to be sorted before it reaches the degradative lysosome. For example, low-density lipoprotein (LDL) is taken into the cell by binding to the LDL receptor at the cell surface. Upon reaching early endosomes, the LDL dissociates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TMPRSS2
Transmembrane protease, serine 2 is an enzyme that in humans is encoded by the ''TMPRSS2'' gene. It belongs to the TMPRSS family of proteins, whose members are transmembrane proteins which have a serine protease activity. The TMPRSS2 protein is found in high concentration in the cell membranes of epithelial cells of the lung and of the prostate, but also in the heart, liver and gastrointestinal tract. Mutations of the ''TMPRSS2'' gene are often involved in prostate cancer. Several viruses, including SARS-CoV-2, use the protease activity of the TMPRSS2 protein in the process of entering cells. Function The ''TMPRSS2'' gene encodes a protein that belongs to the serine protease family. The encoded protein contains a type II transmembrane domain, a receptor class A domain, a scavenger receptor cysteine-rich domain and a protease domain. Serine proteases are known to be involved in many physiological and pathological processes. This gene is up-regulated by androgenic hormones in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine protease ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |