|
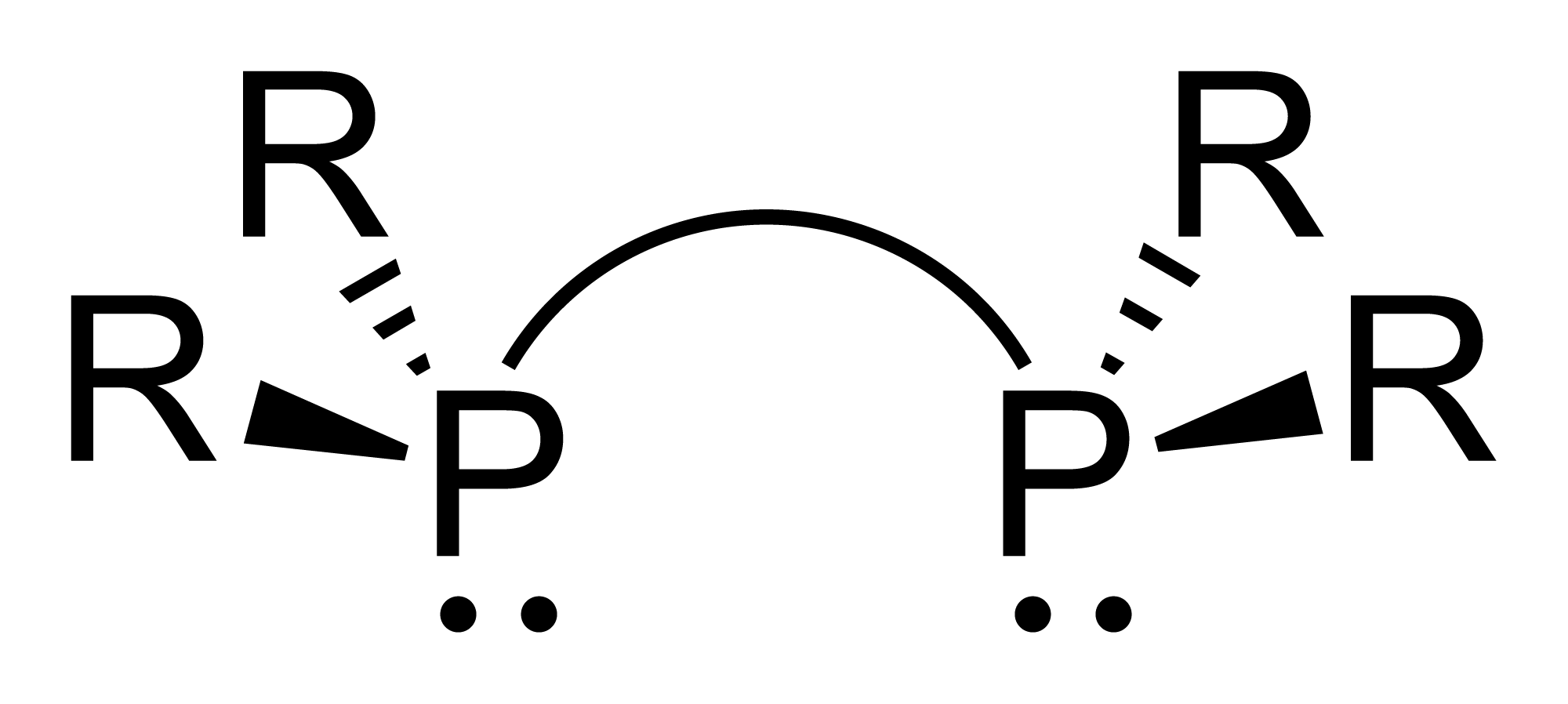
Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts. Synthesis 222px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl + 2 NaPPh2 → Ph2P(CH2)nPPh2 + 2 NaCl Diphosphine ligands can also be prepare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dcpe
Bis(dicyclohexylphosphino)ethane, abbreviated dcpe, is an organophosphorus compound with the formula (C6H11)2PCH2CH2P(C6H11)2. It is a white solid that is soluble in nonpolar organic solvents. The compound is used as a bulky and highly basic diphosphine ligand in coordination chemistry A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ....Sowa, J. R., Jr., Zanotti, V., Facchin, G., Angelici, R. J., "Calorimetric studies of the heats of protonation of the metal in Fe(CO)3(bidentate phosphine, arsine) complexes: effects of chelate ligands on metal basicity", J. Am. Chem. Soc. 1992, 114, 160. References {{DEFAULTSORT:Bis(dicyclohexylphosphino)ethane, 1,2- Chelating agents Diphosphines 1,2-Ethanediyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1-Bis(diphenylphosphino)methane
1,1-Bis(diphenylphosphino)methane (dppm), is an organophosphorus compound with the formula CH2(PPh2)2. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand. It is more specifically a chelating ligand because it is a ligand that can bond to metals with two phosphorus donor atoms. The natural bite angle is 73°. Synthesis and reactivity 1,1-Bis(diphenylphosphino)methane was first prepared by the reaction of sodium diphenylphosphide (Ph2PNa) with dichloromethane: :Ph3P + 2 Na → Ph2PNa + NaPh :2NaPPh2 + CH2Cl2 → Ph2PCH2PPh2 + 2 NaCl The methylene group (CH2) in dppm (and especially its complexes) is mildly acidic. The ligand can be oxidized to give the corresponding oxides and sulfides CH2 (E)Ph2sub>2 (E = O, S). The methylene group is even more acidic in these derivatives. Coordination chemistry As a chelating ligand, 1,1-bis(diphenylphosphino)methane forms a four-membered ring with the constituents MP2C. The ligand promotes the format ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine Ligand
A metal-phosphine complex is a In coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0). Preparation Many metal phosphine complexes are prepared by reactions of metal halides with preformed phosphines. For example, treatment of a suspension of palladium chloride in ethanol with triphenylphosphine yields monomeric bis(triphenylphosphine)palladium(II) chloride units. : dCl2sub>n + 2PPh3 → PdCl2(PPh3)2 The first reported phosphine complexes were ''cis''- and ''trans''-PtCl2(PEt3)2 reported by Cahours and Gal in 1870. Often the phosphine serves both as a ligand and as a reductant. This property is illustrated by the synthesis of many platinum-metal complexes of triphenylph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bis(dicyclohexylphosphino)ethane
Bis(dicyclohexylphosphino)ethane, abbreviated dcpe, is an organophosphorus compound with the formula (C6H11)2PCH2CH2P(C6H11)2. It is a white solid that is soluble in nonpolar organic solvents. The compound is used as a bulky and highly basic diphosphine ligand in coordination chemistry A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ....Sowa, J. R., Jr., Zanotti, V., Facchin, G., Angelici, R. J., "Calorimetric studies of the heats of protonation of the metal in Fe(CO)3(bidentate phosphine, arsine) complexes: effects of chelate ligands on metal basicity", J. Am. Chem. Soc. 1992, 114, 160. References {{DEFAULTSORT:Bis(dicyclohexylphosphino)ethane, 1,2- Chelating agents Diphosphines 1,2-Ethanediyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DIPAMP
DIPAMP is an organophosphorus compound that is used as a ligand in homogeneous catalysis. It is a white solid that dissolves in organic solvents. Work on this compound by W. S. Knowles was recognized with the Nobel Prize in Chemistry. DIPAMP was the basis for of the first industrial scale asymmetric hydrogenation, the synthesis of the drug L-DOPA. : DIPAMP is a C2-symmetric diphosphine. Each phosphorus Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ... centre, which is pyramidal, bears three different substituents - anisyl, phenyl, and the ethylene group. The ligand therefore exists as the enantiomeric (''R'',''R'') and (''S'',''S'') pair, as well as the achiral ''meso'' isomer. DIPAMP was originally prepared by an oxidative coupling, starting from anisyl(phenyl)(methyl)phosph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DPPE Structure
1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (PhPCH) (Ph = phenyl). It is a commonly used bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents. Preparation The preparation of dppe is by the alkylation of NaPPh: :P(CH) + 2 Na → NaP(CH) + NaCH NaP(CH), which is readily air-oxidized, is treated with 1,2-dichloroethane (ClCHCHCl) to give dppe: :2 NaP(CH) + ClCHCHCl → (CH)PCHCHP(CH) + 2 NaCl Reactions The reduction of dppe by lithium to give PhHP(CH)PHPh has been reported. :PhP(CH)PPh + 4 Li → PhLiP(CH)PLiPh + 2 PhLi Hydrolysis gives the bis(secondary phosphine): :PhLiP(CH)PLiPh + 2 PhLi + 4HO → PhHP(CH)PHPh + 4 LiOH + 2 CH : Treatment of dppe with conventional oxidants such as hydrogen peroxide (HO), aqueous bromine (Br), etc., produces dppeO in low yield (e.g., 13%) as a result of non-selective oxidation.Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons, Ltd Select ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dppbz
1,2-Bis(diphenylphosphino)benzene (dppbz) is an organophosphorus compound with the formula C6H4(PPh2)2 ( Ph = C6H5). Classified as a diphosphine ligand, it is a common bidentate ligand in coordination chemistry. It is a white, air-stable solid. As a chelating ligand, dppbz is very similar to 1,2-bis(diphenylphosphino)ethylene cis-1,2-Bis(diphenylphosphino)ethylene (dppv) is an organophosphorus compound with the formula C2H2(PPh2)2 ( Ph = C6H5). Both the cis and trans isomers are known, but the cis isomer is of primary interest. Classified as a diphosphine ligand, it i .... References {{DEFAULTSORT:Bis(diphenylphosphino)benzene, 1,2- Chelating agents Diphosphines Phenyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Bis(diphenylphosphino)benzene
1,2-Bis(diphenylphosphino)benzene (dppbz) is an organophosphorus compound with the formula C6H4(PPh2)2 ( Ph = C6H5). Classified as a diphosphine ligand, it is a common bidentate ligand in coordination chemistry A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many .... It is a white, air-stable solid. As a chelating ligand, dppbz is very similar to 1,2-bis(diphenylphosphino)ethylene. References {{DEFAULTSORT:Bis(diphenylphosphino)benzene, 1,2- Chelating agents Diphosphines Phenyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Bis(diisopropylphosphino)ethane
1,2-Bis(diisopropylphosphino)ethane (dippe) is a commonly used bidentate ligand in coordination chemistry. This compound is similar to the ligand 1,2-bis(diphenylphosphino)ethane 1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (PhPCH) (Ph = phenyl). It is a commonly used bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents. Preparation ... (dppe), with the substitution of isopropyl groups for phenyl groups. {{DEFAULTSORT:Bis(diisopropylphosphino)ethane, 1,2- Diphosphines Isopropyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Bis(dimethylphosphino)ethane
1,2-Bis(dimethylphosphino)ethane (dmpe) is a diphosphine ligand in coordination chemistry. It is a colorless, air-sensitive liquid that is soluble in organic solvents. With the formula (CHPMe), dmpe is used as a compact strongly basic spectator ligand (Me = methyl), Representative complexes include V(dmpe)(BH), Mn(dmpe)(AlH), Tc(dmpe)(CO)Cl, and Ni(dmpe)Cl. Synthesis It is synthesised by the reaction of methylmagnesium iodide with 1,2-bis(dichlorophosphino)ethane: :ClPCHCHPCl + 4 MeMgI → MePCHCHPMe + 4 MgICl Alternatively it can be generated by alkylation of sodium dimethylphosphide. The synthesis of dmpe from thiophosphoryl chloride has led to serious accidents and has been abandoned.} Related ligands * Bis(dicyclohexylphosphino)ethane, a bulkier analogue, which is also a solid. * 1,2-Bis(diphenylphosphino)ethane, more air-stable than dmpe, but less basic. * 1,2-Bis(dimethylphosphino)benzene, a more rigid analogue of dmpe. * Tetramethylethylenediamine Tetrame ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Bis(diphenylphosphino)ethane
1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (PhPCH) (Ph = phenyl). It is a commonly used bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents. Preparation The preparation of dppe is by the alkylation of NaPPh: :P(CH) + 2 Na → NaP(CH) + NaCH NaP(CH), which is readily air-oxidized, is treated with 1,2-dichloroethane (ClCHCHCl) to give dppe: :2 NaP(CH) + ClCHCHCl → (CH)PCHCHP(CH) + 2 NaCl Reactions The reduction of dppe by lithium to give PhHP(CH)PHPh has been reported. :PhP(CH)PPh + 4 Li → PhLiP(CH)PLiPh + 2 PhLi Hydrolysis gives the bis(secondary phosphine): :PhLiP(CH)PLiPh + 2 PhLi + 4HO → PhHP(CH)PHPh + 4 LiOH + 2 CH : Treatment of dppe with conventional oxidants such as hydrogen peroxide (HO), aqueous bromine (Br), etc., produces dppeO in low yield (e.g., 13%) as a result of non-selective oxidation.Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons, Ltd Select ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Bis(diisopropylphosphino)ethane-2D-by-AHRLS-2012
Onekama ( ) is a village in Manistee County in the U.S. state of Michigan. The population was 411 at the 2010 census. The village is located on the shores of Portage Lake and is surrounded by Onekama Township. The town's name is derived from "Ona-ga-maa," an Anishinaabe word which means "singing water." Geography According to the United States Census Bureau, the village has a total area of , all land. The M-22 highway runs through downtown Onekama. History The predecessor of the village of Onekama was the settlement of Portage at Portage Point, first established in 1845, at the western end of Portage, at the outlet of Portage Creek. In 1871, when landowners around the land-locked lake became exasperated with the practices of the Portage Sawmill, they took the solution into their own hands and dug a channel through the narrow isthmus, opening a waterway that lowered the lake by 12 to 14 feet and brought it to the same level as Lake Michigan. When this action dried out Portage ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-from-xtal-3D-ball-stick-hybrid.png)

2.png)