|
Cyclotriveratrylene
Cyclotriveratrylene (CTV) is an organic compound with the formula 6H2(OCH3)2CH2sub>3. It is a white solid that is soluble in organic solvents. The compound is a macrocycle and used in host–guest chemistry as a molecular host. Synthesis The compound can be synthesised from veratrole alcohol upon acid catalysis. : Operating via the same intermediates, an alternative synthesis involves the acid-catalyzed reaction of veratrole and formaldehyde. The method is similar to the reactions that give phenol formaldehyde resins except that the degree of polymerization is moderated by the methoxy substituents. Host–guest behavior CTV has a bowl shaped conformation, giving the molecule C3 symmetry. The ether groups are situated at the upper rim, but in contrast a crown ether typically do not interact with guests. The compound is related to calixarenes in terms of its host–guest properties and its synthesis CTV derivates are known to bind fullerenes and the use of CTV's in the sepa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclotriveratrylene Synthesis
Cyclotriveratrylene (CTV) is an organic compound with the formula 6H2(OCH3)2CH2sub>3. It is a white solid that is soluble in organic solvents. The compound is a macrocycle and used in host–guest chemistry as a molecular host. Synthesis The compound can be synthesised from veratrole alcohol upon acid catalysis. : Operating via the same intermediates, an alternative synthesis involves the acid-catalyzed reaction of veratrole and formaldehyde. The method is similar to the reactions that give phenol formaldehyde resins except that the degree of polymerization is moderated by the methoxy substituents. Host–guest behavior CTV has a bowl shaped conformation, giving the molecule C3 symmetry. The ether groups are situated at the upper rim, but in contrast a crown ether typically do not interact with guests. The compound is related to calixarenes in terms of its host–guest properties and its synthesis CTV derivates are known to bind fullerenes and the use of CTV's ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
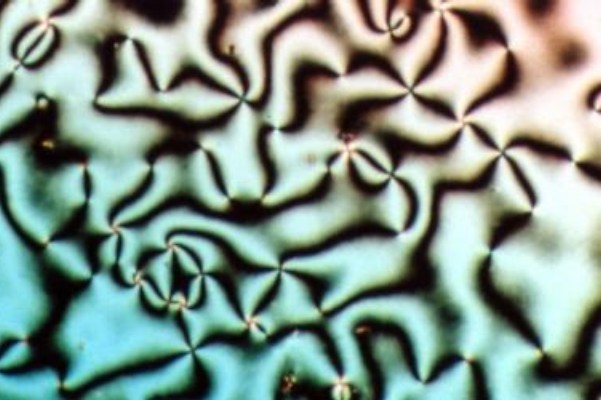
Liquid Crystal
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. LC materials may not always be in a LC state of matter (just as water may be ice or water vapor). Liquid crystals can be divided into 3 main types: * thermotropic, *lyotropic, and * metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions as a function of b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechol Ethers
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It was first discovered by destructive distillation of the plant extract catechin. About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances. Catechol occurs as feathery white crystals that are very rapidly soluble in water. Isolation and synthesis Catechol was first isolated in 1839 by Edgar Hugo Emil Reinsch (1809–1884) by distilling it from the solid tannic preparation catechin, which is the residuum of catechu, the boiled or concentrated juice of ''Mimosa catechu'' (''Acacia catechu''). Upon heating catechin above its decomposition point, a substance that Reinsch first named ''Brenz-Katechusäure'' (burned catechu acid) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrocycles
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. Synthesis The formation of macrocycles by ring-closure is called macrocylization. Pioneering work was reported for studies on terpenoid macrocycles. The central challenge to macrocyclization is that ring-closing reactions do not favor the formation of large rings. Instead, small rings or polymers tend to form. This kinetic problem can be addressed by using high-dilution reactions, whereby intramolecular processes are favored relative to polymerizations. Some macrocyclizations are favored using template reactions. Templates are ions, molecules, surfaces etc. that bind and pre-organize compounds, guiding them toward formation of a particular ring size. The crown ethers are often generated in the presence of an alkali metal cation, whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gertrude Maud Robinson
Gertrude Maud Robinson (formerly Walsh) was an influential organic chemist most famous for her work on plant pigments; the Piloty-Robinson Pyrrole Synthesis, which is named for her; her syntheses of fatty acids; and her synthesis of δ-hexenolactone,Medawar, P.B.; Robinson, G.M.; Robinson, R. A Synthetic Differential Growth Inhibitor. ''Nature'', 1943, ''151'', 195. the first synthetic molecule with the character of penicillin.Dunstan, A.E.; Woodhead, D.W.; Simonsen, J.L. Obituary notices. ''J. Chem. Soc. '', 1954, 2664–2668. Biography Robinson was born on 6 February 1886 in Winsford, Cheshire and died of a heart attack on 1 March 1954. After attending Verdin Secondary School, she was granted her B. Sc. in 1907 and M. Sc. in 1908 from Owens College. She then researched at the University of Manchester under Chaim Weizmann, who later became the first president of Israel, and taught chemistry at the Manchester High School for Girls. In 1912 she married Robert Robinson, who ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Encapsulation
In supramolecular chemistry, molecular encapsulation is the confinement of a guest molecule inside the cavity of a supramolecular host molecule (molecular capsule, molecular container or cage compounds). Examples of supramolecular host molecule include carcerands and endohedral fullerenes. Reactivity of guests An important implication of encapsulation is that the guest behaves differently from the way it would when in solution. The guest molecule tends to be unreactive and often has distinctive spectroscopic signatures. Compounds normally highly unstable in solution, such as arynes or cycloheptatetraene, have been isolated at room temperature when molecularly encapsulated. Examples One of the first examples of encapsulating a structure at the molecular level was demonstrated by Donald Cram and coworkers; they were able to isolate highly unstable, antiaromatic cylobutadiene at room temperature by encapsulating it within a hemicarcerand. Isolation of cyclobutadiene allowed chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cryptophane
Cryptophanes are a class of organic supramolecular compounds studied and synthesized primarily for molecular encapsulation and recognition. One possible noteworthy application of cryptophanes is encapsulation and storage of hydrogen gas for potential use in fuel cell automobiles. Cryptophanes can also serve as containers in which organic chemists can carry out reactions that would otherwise be difficult to run under normal conditions. Due to their unique molecular recognition properties, cryptophanes also hold great promise as a potentially new way to study the binding of organic molecules with substrates, particularly as pertaining to biological and biochemical applications. Discovery Cryptophanes were discovered by André Collet and Jacqueline Gabard in 1981 when these researchers created, using template-directed synthesis, the first cryptophane, now known as cryptophane-A. Structure Cryptophane cages are formed by two cup-shaped .1.1��orthocyclophane units (see cyclotriver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Spacer
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calixarene
A calixarene is a macrocycle or cyclic oligomer based on a methylene-linked phenols. With hydrophobic cavities that can hold smaller molecules or ions, calixarenes belong to the class of cavitands known in host–guest chemistry. Nomenclature Calixarene nomenclature is straightforward and involves counting the number of repeating units in the ring and including it in the name. A calix rene has 4 units in the ring and a calix rene has 6. A substituent in the meso position Rb is added to the name with a prefix C- as in C-methylcalix rene The word calixarene is derived from the Greek calix or chalice because this type of molecule resembles a vase (or cup) and from the word arene that refers to the aromatic building block. Synthesis Calixarenes are generally produced by condensation of two components: an electron-rich aromatic compound, classically a 4-substituted phenol, and an aldehyde, classically formaldehyde. *The scope for the aromatic component is broad diverse. The key at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)

