Gertrude Maud Robinson on:
[Wikipedia]
[Google]
[Amazon]
Gertrude Maud Robinson (formerly Walsh) was an influential
 In 1912 she married Robert Robinson, who later won the 1947
In 1912 she married Robert Robinson, who later won the 1947  She also independently suggested the asymmetric structure of aromatic azoxy-compounds and, with her husband, postulated a mechanism for the
She also independently suggested the asymmetric structure of aromatic azoxy-compounds and, with her husband, postulated a mechanism for the


 There are, however, a few problems with some syntheses. The Piloty-Robinson reaction competes with the formation of
There are, however, a few problems with some syntheses. The Piloty-Robinson reaction competes with the formation of



 One example of this is the synthesis of 10-ketotridecoic acid via 13-diketopalmitic acid, which is an important acid because, with reduction and dehydration, it becomes the molecule that is an active ovarian hormone.
Gertrude Robinson, using her methods for the synthesis of higher fatty acids, synthesized n-triacontanoic acid, also known as Melissic acid, and 13-oxodotetracontanoic acid.Robinson, G.M. ''J. Chem. Soc.'', 1934, 1543-1545.
One example of this is the synthesis of 10-ketotridecoic acid via 13-diketopalmitic acid, which is an important acid because, with reduction and dehydration, it becomes the molecule that is an active ovarian hormone.
Gertrude Robinson, using her methods for the synthesis of higher fatty acids, synthesized n-triacontanoic acid, also known as Melissic acid, and 13-oxodotetracontanoic acid.Robinson, G.M. ''J. Chem. Soc.'', 1934, 1543-1545.

organic chemist
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
most famous for her work on plant pigments; the Piloty-Robinson Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-meth ...
Synthesis, which is named for her; her syntheses of fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, fr ...
s; and her synthesis of δ-hexenolactone,Medawar, P.B.; Robinson, G.M.; Robinson, R. A Synthetic Differential Growth Inhibitor. ''Nature'', 1943, ''151'', 195. the first synthetic molecule with the character of penicillin
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from ''Penicillium'' moulds, principally '' P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum using ...
.Dunstan, A.E.; Woodhead, D.W.; Simonsen, J.L. Obituary notices. ''J. Chem. Soc. '', 1954, 2664–2668.
Biography
Robinson was born on 6 February 1886 in Winsford, Cheshire and died of a heart attack on 1 March 1954. After attending Verdin Secondary School, she was granted her B. Sc. in 1907 and M. Sc. in 1908 from Owens College. She then researched at the University of Manchester underChaim Weizmann
Chaim Azriel Weizmann ( he, חיים עזריאל ויצמן ', russian: Хаим Евзорович Вейцман, ''Khaim Evzorovich Veytsman''; 27 November 1874 – 9 November 1952) was a Russian-born biochemist, Zionist leader and Israel ...
, who later became the first president of Israel, and taught chemistry at the Manchester High School for Girls.
 In 1912 she married Robert Robinson, who later won the 1947
In 1912 she married Robert Robinson, who later won the 1947 Nobel Prize
The Nobel Prizes ( ; sv, Nobelpriset ; no, Nobelprisen ) are five separate prizes that, according to Alfred Nobel's will of 1895, are awarded to "those who, during the preceding year, have conferred the greatest benefit to humankind." Alfr ...
and with whom she coauthored many papers, and moved to the position of an unpaid demonstrator at the University of Sydney
The University of Sydney (USYD), also known as Sydney University, or informally Sydney Uni, is a public research university located in Sydney, Australia. Founded in 1850, it is the oldest university in Australia and is one of the country's si ...
Rayner-Canham, M.; Rayner-Canham, G. ''Chemistry Was Their Life: Pioneering British Women Chemists, 1880-1949'', Imperial College Press: London, 2008. 435-438. before briefly going to the St. Andrews in Scotland and University College
In a number of countries, a university college is a college institution that provides tertiary education but does not have full or independent university status. A university college is often part of a larger university. The precise usage varies ...
in London. She worked on the syntheses of saturated and unsaturated fatty acids and was the first to synthesize oleic acid
Oleic acid is a fatty acid that occurs naturally in various animal and vegetable fats and oils. It is an odorless, colorless oil, although commercial samples may be yellowish. In chemical terms, oleic acid is classified as a monounsaturated omega ...
and lactarinic acid. Her methods led to her synthesis of fatty acids with the greatest molecular weights of the time (specifically, tricontanoic and 13-oxodotetracontanoic acids).
Fischer Indole Synthesis
The Fischer indole synthesis is a chemical reaction that produces the aromatic heterocycle indole from a (substituted) phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Emil Fischer. Today ...
. Based on this mechanism and working off the pyrrole syntheses of Piloty, the couple provided a method for synthesizing tetraphenylpyrrole . The Piloty-Robinson Pyrrole Synthesis is named in their honor.Olson, J.A.; Shea, K.M. ''Acc. Chem. Res.'', 2011, ''44''(5), 311–321.
After moving to the University of Oxford
, mottoeng = The Lord is my light
, established =
, endowment = £6.1 billion (including colleges) (2019)
, budget = £2.145 billion (2019–20)
, chancellor ...
, Gertrude Robinson began studying plant pigments and published extensively on anthocyanin
Anthocyanins (), also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compo ...
s with her husband. Ogilvie, M.; Harvey, J. ''The Biographical Dictionary of Women in Science'', Stratford Publishing: New York, 2000. She was the first to observe that the color of a plant’s pigment was not related to the pH of its sap and she pioneered work in leucoanthocyanins. Additionally, she was the first to synthesize δ-hexenolactone, a molecule similar to penicillin that had its antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
properties. In 1953, the University of Oxford granted her an honorary M.A. degree.
Besides her work as a chemist, Gertrude Robinson had two children, Marion in 1921 and Michael in 1926. She was an avid mountain climber, a prolific traveler, and a frequent hostess. Perhaps inspiring her work on plant pigments, she and her husband also kept a garden for many years.
Plant Genetics
Anthocyanins and Copigments
Flowers, fruits, and leaves get their pigments from anthocyanins and copigments (such as tannins andflavonols
Flavonols are a class of flavonoids that have the 3-hydroxyflavone backbone (IUPAC name : 3-hydroxy-2-phenylchromen-4-one). Their diversity stems from the different positions of the phenols, phenolic hydroxyl, -OH groups. They are distinct from f ...
). The combinations provide the exact colors of various plants at different stages of development.Robinson, G.M. ''J. Chem. Soc.'', 1939, ''61'', 1606-1607. The Robinsons found that, at different ratios of anthocyanins to copigments, the copigments had different effects and they postulated that this was due to the copigments breaking up the anthocyanin complexes, which they observed when they were in solution together.Robinson, G.M.; Robinson, R. ''Biochem.'', 1934, 1687-1720. They studied these pigments by comparing color distributions in immiscible
Miscibility () is the property of two chemical substance, substances to mix in all mixing ratio, proportions (that is, to fully dissolution (chemistry), dissolve in each other at any concentration), forming a homogeneity and heterogeneity, homoge ...
solutions after reactions with alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
s or ferric chloride
Iron(III) chloride is the inorganic compound with the formula . Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The colo ...
.Robinson, G.M.; Robinson, R. ''Biochem.'', 1931, 1687-1705.
Leucoanthocyanins
The Robinsons investigated the structure of leucoanthocyanins, colorless molecules that generate anthocyanidins and are present in most plants. Rosenheim simultaneously discovered leucoanthocyanins and he coined the term.Robinson, G.M.; Robinson, R. ''Biochem.'', 1932, 206-212. Leucoanthocyanins occur in more locations (wood, bark, nutshells, flowers, fruits) than normal anthocyanins.Lawrence, W. J. C.; Price, J.R.; Robinson, G.M.; Robinson, R. ''Biochem.'', 1938, 1661-1667.
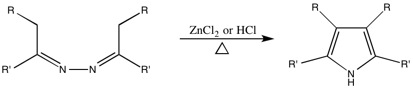
Piloty-Robinson Pyrrole Synthesis
This reaction, originally named after Piloty, had the Robinson name added to it due to their work on the mechanism. While it is unclear which Robinson the synthesis is technically named after, the paper on the topic was authored by both Gertrude and Robert.Generalized Synthesis
This reaction is used to convertazine
Azines are a functional class of organic compounds with the connectivity RR'C=N-N=CRR'. These compounds are the product of the condensation of hydrazine with ketones and aldehydes, although in practice they are often made by alternative routes ...
s to 3,4-disubstituted pyrroles.


Generalized Mechanism
The mechanism as suggested by the Robinsons.Robinson, G.M.; Robinson, R. ''J. Chem. Soc., Trans.'', 1918, ''113'', 639-645.Wang, Z. ''Comprehensive Organic Name Reactions and Reagents'', Wiley: Hoboken, 2010.Mundy, B.P.; Ellerd, M.G.; Favaloro, F.G. ''Name Reactions and Reagents in Organic Synthesis'', 2nd ed.; Wiley: Hoboken, 2005, 510-511. There are, however, a few problems with some syntheses. The Piloty-Robinson reaction competes with the formation of
There are, however, a few problems with some syntheses. The Piloty-Robinson reaction competes with the formation of pyrazoline
Pyrazoline is a heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with ...
when the reactant is an aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or ...
azine derived from a ketone. Also, under high temperatures and highly acidic solutions, azines derived from aldehydes are not stable. This prevents the formation of 2,5-disubstituted pyrroles (where R=H) using this method.Leeper, F.J.; Kelly, J.M. ''Organic Preparations and Procedures International: The New Journal for Organic Synthesis'', 2013, ''45:3'', 171-210.
Modern Uses
While the pyrroles produced by the Piloty-Robinson Synthesis are often very useful, the reaction itself is not always favorable because it requires high temperatures and long reaction times in addition to the problems mentioned above, the yield is often low or moderate.Milgram, B.C.; Eskildsen, K.; Richter, S.M.; Scheidt, W.R.; Scheidt, K. A. ''J. Org. Chem.'', 2007, ''72'', 3941-3944. Modern methods have alleviated some of these concerns.Microwave Irradiation
Microwave radiation
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequencies between 300 MHz and 300 GHz respectively. Different sources define different frequency rang ...
decreases the time necessary for the reaction from around 3 days to 30-60 min. It can also affect the yield.

Solid-Supported
Solid-supported syntheses offer an easier and more efficient workup and purification.Tanaka, H..; Moriwaki, M.; Takahashi, T. ''Org. Lett.'', 2003, ''5'', 3807-3809.
Fischer Indole Mechanism
The Robinsons disproved many of the prevailing theories about the Fischer Indole Mechanism by showing that the reaction went unperturbed in the presence of otheraromatic amine
In organic chemistry, an aromatic amine is an organic compound consisting of an aromatic ring attached to an amine. It is a broad class of compounds that encompasses anilines, but also many more complex aromatic rings and many amine substituents ...
s such as p-toluidine
There are three isomers of toluidine, which are organic compounds. These isomers are ''o''-toluidine, ''m''-toluidine, and ''p''-toluidine, with the prefixed letter abbreviating, respectively, ''ortho''; ''meta''; and ''para''. All three are aryl ...
. This is the mechanism they suggested (where hydrogen shifts may also be interpreted as hydrogen exchanges in acid).

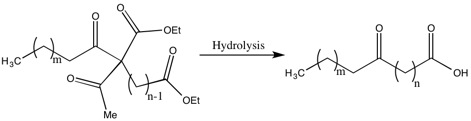
Saturated and Unsaturated Fatty Acids
Methods of Synthesis of Higher Fatty Acids
One of the drawbacks of the Robinsons’ methods for the synthesis of fatty acids are the low yields due to the recoveries of a significant portion of the dialdehyde. The justification by Gertrude Robinson for this low yield was that thealdehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
intermediate was a weaker acid than acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
, which was removed during a step in the hydrolysis. While she did not solve this problem, she did improve the yield and decrease the dialdehyde recovered by “the acylation of a substituted ethyl acetoacetate by the group related to the weakest possible acid”.Robinson, G.M. ''J. Chem. Soc.'', 1930, 745-751.
 One example of this is the synthesis of 10-ketotridecoic acid via 13-diketopalmitic acid, which is an important acid because, with reduction and dehydration, it becomes the molecule that is an active ovarian hormone.
Gertrude Robinson, using her methods for the synthesis of higher fatty acids, synthesized n-triacontanoic acid, also known as Melissic acid, and 13-oxodotetracontanoic acid.Robinson, G.M. ''J. Chem. Soc.'', 1934, 1543-1545.
One example of this is the synthesis of 10-ketotridecoic acid via 13-diketopalmitic acid, which is an important acid because, with reduction and dehydration, it becomes the molecule that is an active ovarian hormone.
Gertrude Robinson, using her methods for the synthesis of higher fatty acids, synthesized n-triacontanoic acid, also known as Melissic acid, and 13-oxodotetracontanoic acid.Robinson, G.M. ''J. Chem. Soc.'', 1934, 1543-1545.

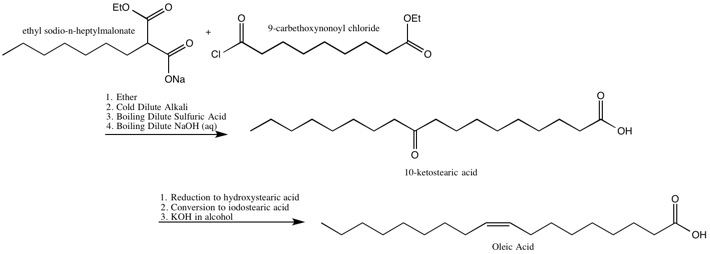
Oleic Acid
The Robinsons identified the location of the double bond in, and also synthesized,oleic acid
Oleic acid is a fatty acid that occurs naturally in various animal and vegetable fats and oils. It is an odorless, colorless oil, although commercial samples may be yellowish. In chemical terms, oleic acid is classified as a monounsaturated omega ...
.
The Robinsons’ Synthesis of Oleic Acid

Lactarinic Acid
Lactarinic Acid, isolated from fungi of theLactarius
''Lactarius'' is a genus of mushroom-producing, ectomycorrhizal fungi, containing several edible species. The species of the genus, commonly known as milk-caps, are characterized by the milky fluid ("latex") they exude when cut or damaged. Like ...
genus
Genus ( plural genera ) is a taxonomic rank used in the biological classification of extant taxon, living and fossil organisms as well as Virus classification#ICTV classification, viruses. In the hierarchy of biological classification, genus com ...
, was shown to contain a ketostearic acid.''Nature'', 1911, ''87'', 442. The Robinsons showed that this was in fact 6-ketostearic acid by doing a Beckmann TransformationSluiter, C.H.; Lobry de Bruyn, C.A. ''KNAW, Proceedings'', 1904, ''6'', 773-778. on the oxime
In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted ...
of lactarinic acid. They then synthesized 6-ketostearic acid via a reaction of ethyl sodio-2-acetyl-n-tridecoate and 5-carbethoxyvaleryl chloride and then hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
to prove the structure of lactarinic acid.Robinson, G.M.; Robinson, R. ''J. Chem. Soc.'', 1925, ''127'', 175-180.
Notes
References
{{DEFAULTSORT:Robinson, Gertrude Maud 1886 births 1954 deaths English chemists British women chemists Organic chemists Alumni of the Victoria University of Manchester People from Winsford 20th-century British women scientists