|
Cyclin A2
Cyclin-A2 is a protein that in humans is encoded by the ''CCNA2'' gene. It is one of the two types of cyclin A: cyclin A1 is expressed during meiosis and embryogenesis while cyclin A2 is expressed in dividing somatic cells. Function Cyclin A2 belongs to the cyclin family, whose members regulate cell cycle progression by interacting with CDK kinases. Cyclin A2 is unique in that it can activate two different CDK kinases; it binds CDK2 during S phase, and CDK1 during the transition from G2 to M phase. Cyclin A2 is synthesized at the onset of S phase and localizes to the nucleus, where the cyclin A2-CDK2 complex is implicated in the initiation and progression of DNA synthesis. Phosphorylation of CDC6 and MCM4 by the cyclin A2-CDK2 complex prevents re-replication of DNA during the cell cycle. Cyclin A2 is involved in the G2/M transition but it cannot independently form a maturation promoting factor (MPF). Recent studies have shown that the cyclin A2-CDK1 complex triggers cyclin B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid resid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flap Structure-specific Endonuclease 1
Flap endonuclease 1 is an enzyme that in humans is encoded by the ''FEN1'' gene. Function The protein encoded by this gene removes 5' overhanging "flaps" (or short sections of single stranded DNA that "hang off" because their nucleotide bases are prevented from binding to their complementary base pair—despite any base pairing downstream) in DNA repair and processes the 5' ends of Okazaki fragments in lagging strand DNA synthesis. Direct physical interaction between this protein and AP endonuclease 1 during long-patch base excision repair provides coordinated loading of the proteins onto the substrate, thus passing the substrate from one enzyme to another. The protein is a member of the XPG/RAD2 endonuclease family and is one of ten proteins essential for cell-free DNA replication. DNA secondary structure can inhibit flap processing at certain trinucleotide repeats in a length-dependent manner by concealing the 5' end of the flap that is necessary for both binding and cleavage ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
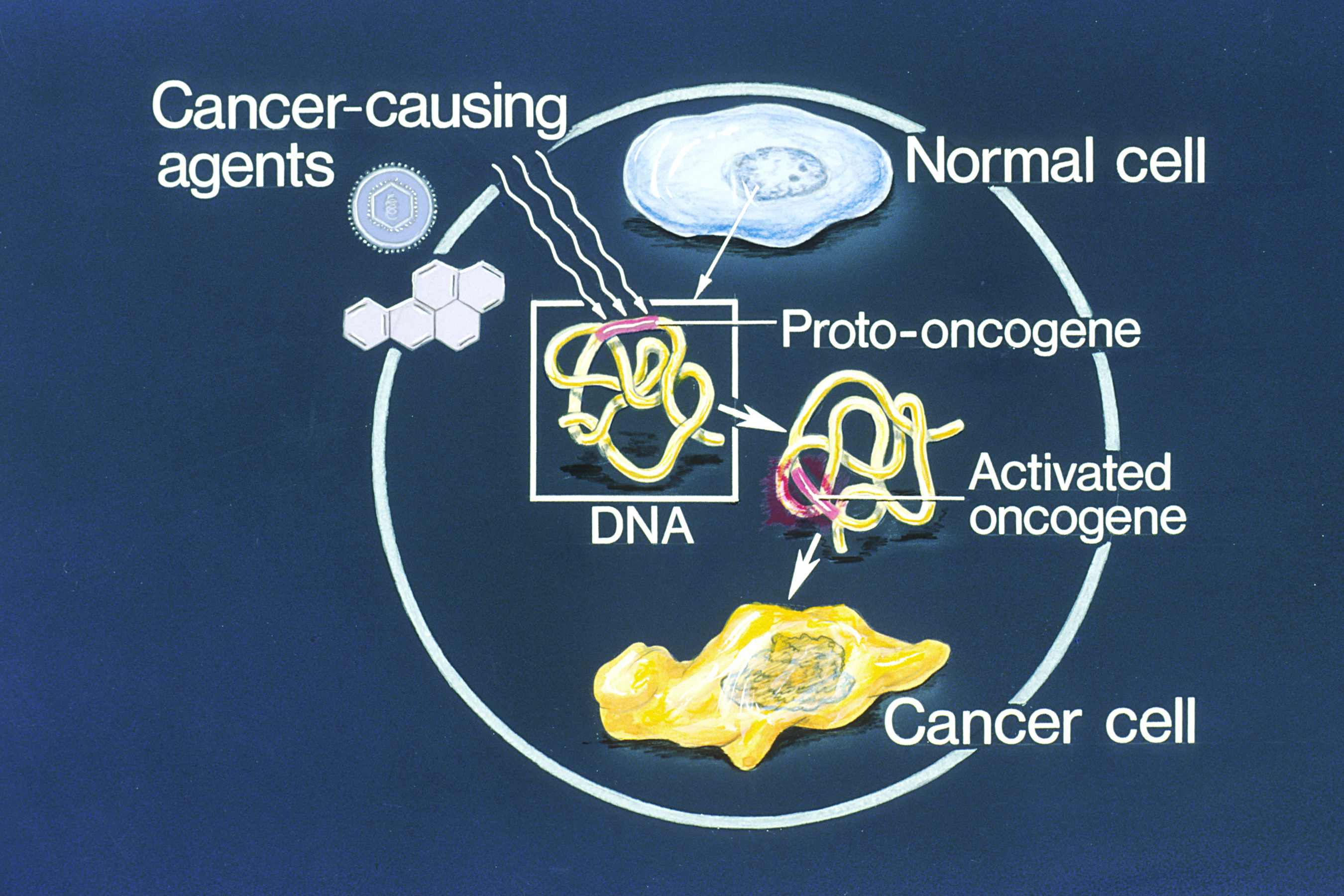
Oncogene
An oncogene is a gene that has the potential to cause cancer. In tumor cells, these genes are often mutated, or expressed at high levels.Kimball's Biology Pages. "Oncogenes" Free full text Most normal cells will undergo a programmed form of rapid cell death ( apoptosis) when critical functions are altered and malfunctioning. Activated oncogenes can cause those cells designated for apoptosis to survive and proliferate instead. Most oncogenes began as proto-oncogenes: normal genes involved in cell growth and proliferation or inhibition of apoptosis. If, through mutation, normal genes promoting cellular growth are up-regulated (gain-of-function mutation), they will predispose the cell to cancer; thus, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anaphase
Anaphase () is the stage of mitosis after the process of metaphase, when replicated chromosomes are split and the newly-copied chromosomes (daughter chromatids) are moved to opposite poles of the cell. Chromosomes also reach their overall maximum condensation in late anaphase, to help chromosome segregation and the re-formation of the nucleus. Anaphase starts when the anaphase promoting complex marks an inhibitory chaperone called securin for destruction by ubiquinylating it. Securin is a protein which inhibits a protease known as separase. The destruction of securin unleashes separase which then breaks down cohesin, a protein responsible for holding sister chromatids together. At this point, three subclasses of microtubule unique to mitosis are involved in creating the forces necessary to separate the chromatids: kinetochore microtubules, interpolar microtubules, and astral microtubules. The centromeres are split, and the sister chromatids are pulled toward the poles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metaphase
Metaphase ( and ) is a stage of mitosis in the eukaryotic cell cycle in which chromosomes are at their second-most condensed and coiled stage (they are at their most condensed in anaphase). These chromosomes, carrying genetic information, align in the equator of the cell before being separated into each of the two daughter cells. Metaphase accounts for approximately 4% of the cell cycle's duration. Preceded by events in prometaphase and followed by anaphase, microtubules formed in prophase have already found and attached themselves to kinetochores in metaphase. In metaphase, the centromeres of the chromosomes convene themselves on the ''metaphase plate'' (or ''equatorial plate''), an imaginary line that is equidistant from the two centrosome poles. This even alignment is due to the counterbalance of the pulling powers generated by the opposing kinetochore microtubules, analogous to a tug-of-war between two people of equal strength, ending with the destruction of B cyclin. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
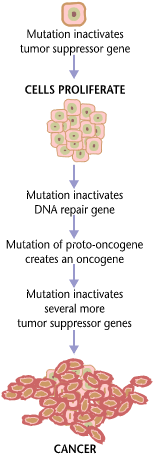
Tumorigenesis
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnormal cell division. Cell division is a physiological process that occurs in almost all tissues and under a variety of circumstances. Normally, the balance between proliferation and programmed cell death, in the form of apoptosis, is maintained to ensure the integrity of tissues and organs. According to the prevailing accepted theory of carcinogenesis, the somatic mutation theory, mutations in DNA and epimutations that lead to cancer disrupt these orderly processes by interfering with the programming regulating the processes, upsetting the normal balance between proliferation and cell death. This results in uncontrolled cell division and the evolution of those cells by natural selection in the body. Only certain mutations lead to can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteasome
Proteasomes are protein complexes which degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases. Proteasomes are part of a major mechanism by which cells regulate the concentration of particular proteins and degrade misfolded proteins. Proteins are tagged for degradation with a small protein called ubiquitin. The tagging reaction is catalyzed by enzymes called ubiquitin ligases. Once a protein is tagged with a single ubiquitin molecule, this is a signal to other ligases to attach additional ubiquitin molecules. The result is a ''polyubiquitin chain'' that is bound by the proteasome, allowing it to degrade the tagged protein. The degradation process yields peptides of about seven to eight amino acids long, which can then be further degraded into shorter amino acid sequences and used in synthesizing new proteins. Proteasomes are found inside all eukaryotes and archaea, and in some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RAD51
DNA repair protein RAD51 homolog 1 is a protein encoded by the gene ''RAD51''. The enzyme encoded by this gene is a member of the RAD51 protein family which assists in repair of DNA double strand breaks. RAD51 family members are homologous to the bacterial RecA, Archaeal RadA and yeast Rad51. The protein is highly conserved in most eukaryotes, from yeast to humans. Variants Two alternatively spliced transcript variants of this gene, which encode distinct proteins, have been reported. Transcript variants utilizing alternative polyA signals exist. Family In mammals, seven recA-like genes have been identified: Rad51, Rad51L1/B, Rad51L2/C, Rad51L3/D, XRCC2, XRCC3, and DMC1/Lim15. All of these proteins, with the exception of meiosis-specific DMC1, are essential for development in mammals. Rad51 is a member of thRecA-like NTPases Function In humans, RAD51 is a 339-amino acid protein that plays a major role in homologous recombination of DNA during double strand brea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MRE11
Double-strand break repair protein MRE11 is an enzyme that in humans is encoded by the ''MRE11'' gene. The gene has been designated ''MRE11A'' to distinguish it from the pseudogene ''MRE11B'' that is nowadays named ''MRE11P1''. Function This gene encodes a nuclear protein involved in homologous recombination, telomere length maintenance, and DNA double-strand break repair. By itself, the protein has 3' to 5' exonuclease activity and endonuclease activity. The protein forms a complex with the RAD50 homolog; this complex is required for nonhomologous joining of DNA ends and possesses increased single-stranded DNA endonuclease and 3' to 5' exonuclease activities. In conjunction with a DNA ligase, this protein promotes the joining of noncomplementary ends in vitro using short homologies near the ends of the DNA fragments. This gene has a pseudogene on chromosome 3. Alternative splicing of this gene results in two transcript variants encoding different isoforms. Orthologs Mre11, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DNA Repair
DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as radiation can cause DNA damage, resulting in tens of thousands of individual molecular lesions per cell per day. Many of these lesions cause structural damage to the DNA molecule and can alter or eliminate the cell's ability to transcribe the gene that the affected DNA encodes. Other lesions induce potentially harmful mutations in the cell's genome, which affect the survival of its daughter cells after it undergoes mitosis. As a consequence, the DNA repair process is constantly active as it responds to damage in the DNA structure. When normal repair processes fail, and when cellular apoptosis does not occur, irreparable DNA damage may occur, including double-strand breaks and DNA crosslinkages (interstrand crosslinks or ICLs). This can eventually lead to malig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homologous Recombination
Homologous recombination is a type of genetic recombination in which genetic information is exchanged between two similar or identical molecules of double-stranded or single-stranded nucleic acids (usually DNA as in cellular organisms but may be also RNA in viruses). Homologous recombination is widely used by cells to accurately DNA repair harmful breaks that occur on both strands of DNA, known as double-strand breaks (DSB), in a process called homologous recombinational repair (HRR). Homologous recombination also produces new combinations of DNA sequences during meiosis, the process by which eukaryotes make gamete cells, like sperm and egg cells in animals. These new combinations of DNA represent genetic variation in offspring, which in turn enables populations to adapt during the course of evolution. Homologous recombination is also used in horizontal gene transfer to exchange genetic material between different strains and species of bacteria and viruses. Horizontal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SKP2
S-phase kinase-associated protein 2 is an enzyme that in humans is encoded by the ''SKP2'' gene. Structure and function Skp2 contains 424 residues in total with the ~40 amino acid F-box domain lying closer to the N-terminal region at the 94-140 position and the C-terminal region forming a concave surface consisting of ten leucine-rich repeats (LRRs). The F-box proteins constitute one of the four subunits of ubiquitin protein ligase complex called SCFs ( SKP1-cullin- F-box), which often—but not always—recognize substrates in a phosphorylation-dependent manner. In this SCF complex, Skp2 acts as the substrate recognition factor. F-box Domain The F-box proteins are divided into three classes: Fbxws containing WD40 repeat domains, Fbxls containing leucine-rich repeats, and Fbxos containing either different protein–protein interaction modules or no recognizable motifs. The protein encoded by this gene belongs to the Fbxls class. In addition to an F-box, this protein contain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |