|
Cyclic Pyranopterin Monophosphate
Fosdenopterin (or cyclic pyranopterin monophosphate, cPMP), sold under the brand name Nulibry, is a medication used to reduce the risk of death due to a rare genetic disease known as molybdenum cofactor deficiency type A. The most common side effects include complications related to the intravenous line, fever, respiratory infections, vomiting, gastroenteritis, and diarrhea. Fosdenopterin was approved for medical use in the United States in February 2021, It is the first medication approved by the U.S. Food and Drug Administration (FDA) for the treatment of molybdenum cofactor deficiency type A. and in the European Union in September 2022. The US Food and Drug Administration considers it to be a first-in-class medication. Medical uses Fosdenopterin is indicated to reduce the risk of mortality in people with molybdenum cofactor deficiency (MoCD) type A. Mechanism of action People with molybdenum cofactor deficiency type A cannot produce cyclic pyranopterin monophosphate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intravenous
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrients for those who cannot, or will not—due to reduced mental states or otherwise—consume food or water by mouth. It may also be used to administer medications or other medical therapy such as blood products or electrolytes to correct electrolyte imbalances. Attempts at providing intravenous therapy have been recorded as early as the 1400s, but the practice did not become widespread until the 1900s after the development of techniques for safe, effective use. The intravenous route is the fastest way to deliver medications and fluid replacement throughout the body as they are introduced directly into the circulatory system and thus quickly distributed. For this reason, the intravenous route of administration is also used for the consu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthine Dehydrogenase
Xanthine dehydrogenase, also known as XDH, is a protein that, in humans, is encoded by the ''XDH'' gene. Function Xanthine dehydrogenase belongs to the group of molybdenum-containing hydroxylases involved in the oxidative metabolism of purines. The enzyme is a homodimer. Xanthine dehydrogenase can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. Xanthine dehydrogenase catalysis, catalyzes the following chemical reaction: xanthine + NAD+ + H2O \rightleftharpoons urate + NADH + H+ The three substrate (biochemistry), substrates of this enzyme are xanthine, nicotinamide adenine dinucleotide, NAD+, and water, H2O, whereas its three product (chemistry), products are urate, nicotinamide adenine dinucleotide, NADH, and hydrogen ion, H+. This enzyme participates in purine metabolism. Nomenclature This enzyme belongs to the family of oxidoreductases, to be specific, those acting on CH or CH2 groups with NAD+ or NAD ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breakthrough Therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "breakthrough therapy" designation is not intended to imply that a drug is actually a "breakthrough" or that there is high-quality evidence of treatment efficacy for a particular condition; rather, it allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA has other mechanisms for expediting the review and approval process for promising drugs, including fast track designation, accelerated approval, and priority review. Requirements A breakthrough therapy designation can be assigned to a drug if "it is a drug which is intended alone or in combinatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Medicinal Products For Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee A committee or commission is a body of one or more persons subordinate to a deliberative assembly. A committee is not itself considered to be a form of assembly. Usually, the assembly sends matters into a committee as a way to explore them more ... responsible for elaborating the agency's opinions on all issues regarding medicinal products for human use. See also * Committee for Medicinal Products for Veterinary Use References External links Committee for Medicinal Products for Human Use (CHMP) Health and the European Union {{eu-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breakthrough Therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "breakthrough therapy" designation is not intended to imply that a drug is actually a "breakthrough" or that there is high-quality evidence of treatment efficacy for a particular condition; rather, it allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA has other mechanisms for expediting the review and approval process for promising drugs, including fast track designation, accelerated approval, and priority review. Requirements A breakthrough therapy designation can be assigned to a drug if "it is a drug which is intended alone or in combinatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Priority Review
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for disease indications with limited profitability. Priority review vouchers are currently earned by pharmaceutical companies for the development and approval of drugs treating neglected tropical diseases, rare pediatric diseases, and "medical countermeasures" for terrorism. The voucher can be used for future drugs that could have wider indications for use, but the company is required to pay a fee (approximately $2.8 million) to use the voucher. When seeking approval for a drug, manufacturers can apply to the FDA for priority review. This is granted when a drug is intended to treat a serious condition and would "provide a sig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Cologne
The University of Cologne (german: Universität zu Köln) is a university in Cologne, Germany. It was established in the year 1388 and is one of the most prestigious and research intensive universities in Germany. It was the sixth university to be established in Central Europe. It closed in 1798 before being re-established in 1919. It is now one of the largest universities in Germany with more than 48,000 students. The University of Cologne was a university of excellence as part of the German Universities Excellence Initiative from 2012 to 2019. As of 2021, 3 Nobel Prize winners have been affiliated with the university. Professors and former students have won 11 Leibniz Prizes, the most prestigious as well as the best-funded prize in Europe. History 1388–1798 The university of Cologne was established in 1388 as the fourth university in the Holy Roman Empire, after the Charles University of Prague (1348), the University of Vienna (1365) and the Ruprecht Karl University ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Braunschweig University Of Technology
Braunschweig () or Brunswick ( , from Low German ''Brunswiek'' , Braunschweig dialect: ''Bronswiek'') is a city in Lower Saxony, Germany, north of the Harz Mountains at the farthest navigable point of the river Oker, which connects it to the North Sea via the rivers Aller and Weser. In 2016, it had a population of 250,704. A powerful and influential centre of commerce in medieval Germany, Brunswick was a member of the Hanseatic League from the 13th until the 17th century. It was the capital city of three successive states: the Principality of Brunswick-Wolfenbüttel (1269–1432, 1754–1807, and 1813–1814), the Duchy of Brunswick (1814–1918), and the Free State of Brunswick (1918–1946). Today, Brunswick is the second-largest city in Lower Saxony and a major centre of scientific research and development. History Foundation and early history The date and circumstances of the town's foundation are unknown. Tradition maintains that Brunswick was created through the me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde Oxidase
Aldehyde oxidase (AO) is a metabolizing enzyme, located in the cytosolic compartment of tissues in many organisms. AO catalyzes the oxidation of aldehydes into carboxylic acid, and in addition, catalyzes the hydroxylation of some heterocycles. It can also catalyze the oxidation of both cytochrome P450 (CYP450) and monoamine oxidase (MAO) intermediate products. AO plays an important role in the metabolism of several drugs. Reaction AO catalyzes the conversion of an aldehyde in the presence of oxygen and water to an acid and hydrogen peroxide. * an aldehyde + H2O + O2 ⇌ a carboxylate + H2O2 + H+ Though the enzyme uses molecular oxygen as an electron acceptor, the oxygen atom that is incorporated into the carboxylate product is from water; however, the exact mechanism of reduction is still not known for AO. The AO also catalyzes the oxidation of heterocycles, which involves a nucleophilic attack located at the carbon atom beside the heteroatom. This means that susceptibility ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
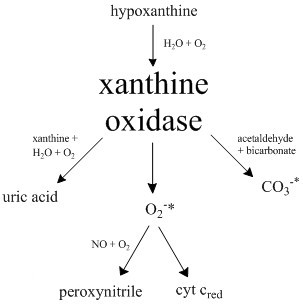
Xanthine Oxidase
Xanthine oxidase (XO, sometimes XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 \rightleftharpoons xanthine + H2O2 * xanthine + H2O + O2 \rightleftharpoons uric acid + H2O2 * Xant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite Oxidase
Sulfite oxidase () is an enzyme in the mitochondria of all eukaryotes, with exception of the yeasts. It oxidizes sulfite to sulfate and, via cytochrome c, transfers the electrons produced to the electron transport chain, allowing generation of ATP in oxidative phosphorylation. This is the last step in the metabolism of sulfur-containing compounds and the sulfate is excreted. Sulfite oxidase is a metallo-enzyme that utilizes a molybdopterin cofactor and a heme group (in a case of animals). It is one of the cytochromes ''b''5 and belongs to the enzyme super-family of molybdenum oxotransferases that also includes DMSO reductase, xanthine oxidase, and nitrite reductase. In mammals, the expression levels of sulfite oxidase is high in the liver, kidney, and heart, and very low in spleen, brain, skeletal muscle, and blood. Structure As a homodimer, sulfite oxidase contains two identical subunits with an N-terminal domain and a C-terminal domain. These two domains are connected b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |