|
Aldehyde Oxidase
Aldehyde oxidase (AO) is a metabolizing enzyme, located in the cytosolic compartment of tissues in many organisms. AO catalyzes the oxidation of aldehydes into carboxylic acid, and in addition, catalyzes the hydroxylation of some heterocycles. It can also catalyze the oxidation of both cytochrome P450 (CYP450) and monoamine oxidase (MAO) intermediate products. AO plays an important role in the metabolism of several drugs. Reaction AO catalyzes the conversion of an aldehyde in the presence of oxygen and water to an acid and hydrogen peroxide. * an aldehyde + H2O + O2 ⇌ a carboxylate + H2O2 + H+ Though the enzyme uses molecular oxygen as an electron acceptor, the oxygen atom that is incorporated into the carboxylate product is from water; however, the exact mechanism of reduction is still not known for AO. The AO also catalyzes the oxidation of heterocycles, which involves a nucleophilic attack located at the carbon atom beside the heteroatom. This means that susceptibility ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde Oxidase 1
Aldehyde oxidase 1 is an enzyme that in humans is encoded by the ''AOX1'' gene. Aldehyde oxidase produces hydrogen peroxide and, under certain conditions, can catalyze the formation of superoxide. Clinical significance Aldehyde oxidase is a candidate gene for amyotrophic lateral sclerosis. See also * MOCOS Molybdenum cofactor sulfurase is an enzyme that in humans is encoded by the ''MOCOS'' gene. MOCOS sulfurates the molybdenum cofactor of xanthine dehydrogenase (XDH) and aldehyde oxidase (AOX1), which is required for their enzymatic activiti ... References External links * Further reading * * * * * * * * * * * * EC 1.2.3 {{gene-2-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are Ribozyme, catalytic RNA molecules, called ribozymes. Enzymes' Chemical specificity, specific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2-Orbital hybridisation, hybridized. The aldehyde group is somewhat polar molecule, polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase
Monoamine oxidases (MAO) () are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase. The MAOs belong to the protein family of flavin-containing amine oxidoreductases. MAOs are important in the breakdown of monoamines ingested in food, and also serve to inactivate monoamine neurotransmitters. Because of the latter, they are involved in a number of psychiatric and neurological diseases, some of which can be treated with monoamine oxidase inhibitors (MAOIs) which block the action of MAOs. Subtypes and tissue distribution In humans there are two types of MAO: MAO-A and MAO-B. * Both are found in neurons and astroglia. * Outside the central nervous system: ** MAO-A is also found in the liver, pulmonary vascular endot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum Flavoprotein
Flavoproteins are proteins that contain a nucleic acid derivative of riboflavin. Flavoproteins are involved in a wide array of biological processes, including removal of radicals contributing to oxidative stress, photosynthesis, and DNA repair. The flavoproteins are some of the most-studied families of enzymes. Flavoproteins have either FMN or FAD (flavin adenine dinucleotide) as a prosthetic group or as a cofactor. The flavin is generally tightly bound (as in adrenodoxin reductase, wherein the FAD is buried deeply). About 5-10% of flavoproteins have a covalently linked FAD. Based on the available structural data, FAD-binding sites can be divided into more than 200 different types. 90 flavoproteins are encoded in the human genome; about 84% require FAD, and around 16% require FMN, whereas 5 proteins require both. Flavoproteins are mainly located in the mitochondria. Of all flavoproteins, 90% perform redox reactions and the other 10% are transferases, lyases, isomerases, ligases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evolutionary Profile
Phylogenetic profiling is a bioinformatics technique in which the joint presence or joint absence of two traits across large numbers of species is used to infer a meaningful biological connection, such as involvement of two different proteins in the same biological pathway. Along with examination of conserved synteny, conserved operon structure, or "Rosetta Stone" domain fusions, comparing phylogenetic profiles is a designated "post-homology" technique, in that the computation essential to this method begins after it is determined which proteins are homologous to which. A number of these techniques were developed by David Eisenberg and colleagues; phylogenetic profile comparison was introduced in 1999 by Pellegrini, ''et al.'' Method Over 2000 species of bacteria, archaea, and eukaryotes are now represented by complete DNA genome sequences. Typically, each gene in a genome encodes a protein that can be assigned to a particular protein family on the basis of homology. For a given ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunosuppressive Drugs
Immunosuppressive drugs, also known as immunosuppressive agents, immunosuppressants and antirejection medications, are drugs that inhibit or prevent activity of the immune system. Classification Immunosuppressive drugs can be classified into five groups: * glucocorticoids * cytostatics * antibodies * drugs acting on immunophilins * other drugs Glucocorticoids In pharmacologic (supraphysiologic) doses, glucocorticoids, such as prednisone, dexamethasone, and hydrocortisone are used to suppress various allergic, inflammatory, and autoimmune disorders. They are also administered as posttransplantory immunosuppressants to prevent the acute transplant rejection and graft-versus-host disease. Nevertheless, they do not prevent an infection and also inhibit later reparative processes. Immunosuppressive mechanism Glucocorticoids suppress cell-mediated immunity. They act by inhibiting genes that code for the cytokines Interleukin 1 (IL-1), IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nrf2
Nuclear factor erythroid 2-related factor 2 (NRF2), also known as nuclear factor erythroid-derived 2-like 2, is a transcription factor that in humans is encoded by the ''NFE2L2'' gene. NRF2 is a basic leucine zipper (bZIP) protein that may regulate the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation, according to preliminary research. In vitro, NRF2 binds to antioxidant response elements (AREs) in the promoter regions of genes encoding cytoprotective proteins. NRF2 induces the expression of heme oxygenase 1 ''in vitro'' leading to an increase in phase II enzymes. NRF2 also inhibits the NLRP3 inflammasome. NRF2 appears to participate in a complex regulatory network and performs a pleiotropic role in the regulation of metabolism, inflammation, autophagy, proteostasis, mitochondrial physiology, and immune responses. Several drugs that stimulate the NFE2L2 pathway are being studied for treatment of diseases that are ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
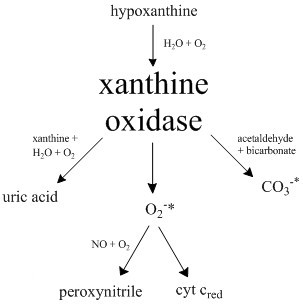
Xanthine Oxidase
Xanthine oxidase (XO, sometimes XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 \rightleftharpoons xanthine + H2O2 * xanthine + H2O + O2 \rightleftharpoons uric acid + H2O2 * Xant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |