|
Cyanoacetic Acid
Cyanoacetic acid is an organic compound. It is a white, hygroscopic solid. The compound contains two functional groups, a nitrile (−C≡N) and a carboxylic acid. It is a precursor to cyanoacrylates, components of adhesives.Harald Strittmatter, Stefan Hildbrand and Peter Pollak Malonic Acid and Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry 2007, Wiley-VCH, Weinheim. Preparation and reactions Cyanoacetic acid is prepared by treatment of chloroacetate salts with sodium cyanide followed by acidification. Electrosynthesis by cathodic reduction of carbon dioxide and anodic oxidation of acetonitrile also affords cyanoacetic acid. Cyanoacetic acid is used to do cyanoacetylation, first convenient method described by J. Slätt.{{cite journal, last1=Bergman, first1=Jan, last2=Romero, first2=Ivan, last3=Slätt, first3=Johnny, title=Cyanoacetylation of indoles, pyrroles and aromatic amines with the combination cyanoacetic acid and acetic anhydride, journal=Synthesis, da ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Cyanoacetate
Ethyl cyanoacetate is an organic compound that contains a carboxylate ester and a nitrile. It is a colourless liquid with a pleasant odor. This material is useful as a starting material for synthesis due to its variety of functional groups and chemical reactivity. Production Ethyl cyanoacetate may be prepared in various ways: * Kolbe nitrile synthesis using ethyl chloroacetate and sodium cyanide. * Fischer esterification of cyanoacetic acid with ethanol in the presence of a strong mineral acids (e.g. concentrated sulfuric acid). The cyanoacetic acid can be prepared via Kolbe nitrile synthesis using sodium chloroacetate and sodium cyanide. *Reaction of the sodium cyanoacetate with ethyl bromide in an aqueous–organic two-phase system in the presence of a phase transfer catalyst. *Oxidation of 3-ethoxypropionitrile, an ether, with oxygen under pressure in the presence of cobalt(II) acetate tetrahydrate as catalyst and ''N''-hydroxyphthalimide as a radical generator. Propertie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LD50
In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a toxic unit that measures the lethal dose of a toxin, radiation, or pathogen. The value of LD50 for a substance is the Dose (pharmacology), dose required to kill half the members of a tested population after a specified test duration. LD50 figures are frequently used as a general indicator of a substance's acute toxicity. A lower LD50 is indicative of increased toxicity. The test was created by J.W. Trevan in 1927. The term semilethal dose is occasionally used in the same sense, in particular with translations of foreign language text, but can also refer to a sublethal dose. LD50 is usually determined by tests on animals such as Laboratory mouse, laboratory mice. In 2011, the U.S. Food and Drug Administration approved alternative methods to LD50 for testing the cosmetic drug Botox without animal tests. Conventions The LD50 is usually expressed as the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allopurinol
Allopurinol is a medication used to decrease high blood uric acid levels. It is specifically used to prevent gout, prevent specific types of kidney stones and for the high uric acid levels that can occur with chemotherapy. It is taken by mouth or injected into a vein. Common side effects when used by mouth include itchiness and rash. Common side effects when used by injection include vomiting and kidney problems. While not recommended historically, starting allopurinol during an attack of gout appears to be safe. In those already on the medication, it should be continued even during an acute gout attack. While use during pregnancy does not appear to result in harm, this use has not been well studied. Allopurinol is in the xanthine oxidase inhibitor family of medications. Allopurinol was approved for medical use in the United States in 1966. It is on the World Health Organization's List of Essential Medicines. Allopurinol is available as a generic medication. In 2020, it was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfadimethoxine
Sulfadimethoxine (or sulphadimethoxine, trade names Di-Methox or Albon) is a long-lasting sulfonamide antimicrobial medication used in veterinary medicine. It is used to treat many infections, including respiratory, urinary tract, enteric, and soft tissue infections and can be given as a standalone or combined with ormetoprim to broaden the target range. Like all sulfamides, sulfadimethoxine inhibits bacterial synthesis of folic acid by acting as a competitive inhibitor against PABA. It is the most common drug prescribed to dogs who have coccidiosis. Mechanism Like other sulfonamides, sulfadimethoxine is a dihydropteroate synthase inhibitor. Bacteria and some protozoa are unable to obtain folic acid from the environment, and must instead synthesize it by converting PABA ( ''para''-aminobenzoate) to dihydropteroate using the enzyme dihydropteroate synthase. Sulfonamides act as a competitive inhibitor; being structurally similar to PABA, they are able to bind to the enzyme's act ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amiloride
Amiloride, sold under the trade name Midamor among others, is a medication typically used with other medications to treat high blood pressure or swelling due to heart failure or cirrhosis of the liver. Amiloride is classified as a potassium-sparing diuretic. Amiloride is often used together with another diuretic, such as a thiazide or loop diuretic. It is taken by mouth. Onset of action is about two hours and it lasts for about a day. Common side effects include high blood potassium, vomiting, loss of appetite, rash, and headache. The risk of high blood potassium is greater in those with kidney problems, diabetes, and those who are older. Amiloride blocks the epithelial sodium channel (ENaC) in the late distal tubule, connecting tubule, and collecting duct of the nephron, which both reduces absorption of sodium ion from the lumen of the nephron and reduces excretion of potassium ion into the lumen. Amiloride was developed in 1967. It is on the World Health Organization's Li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
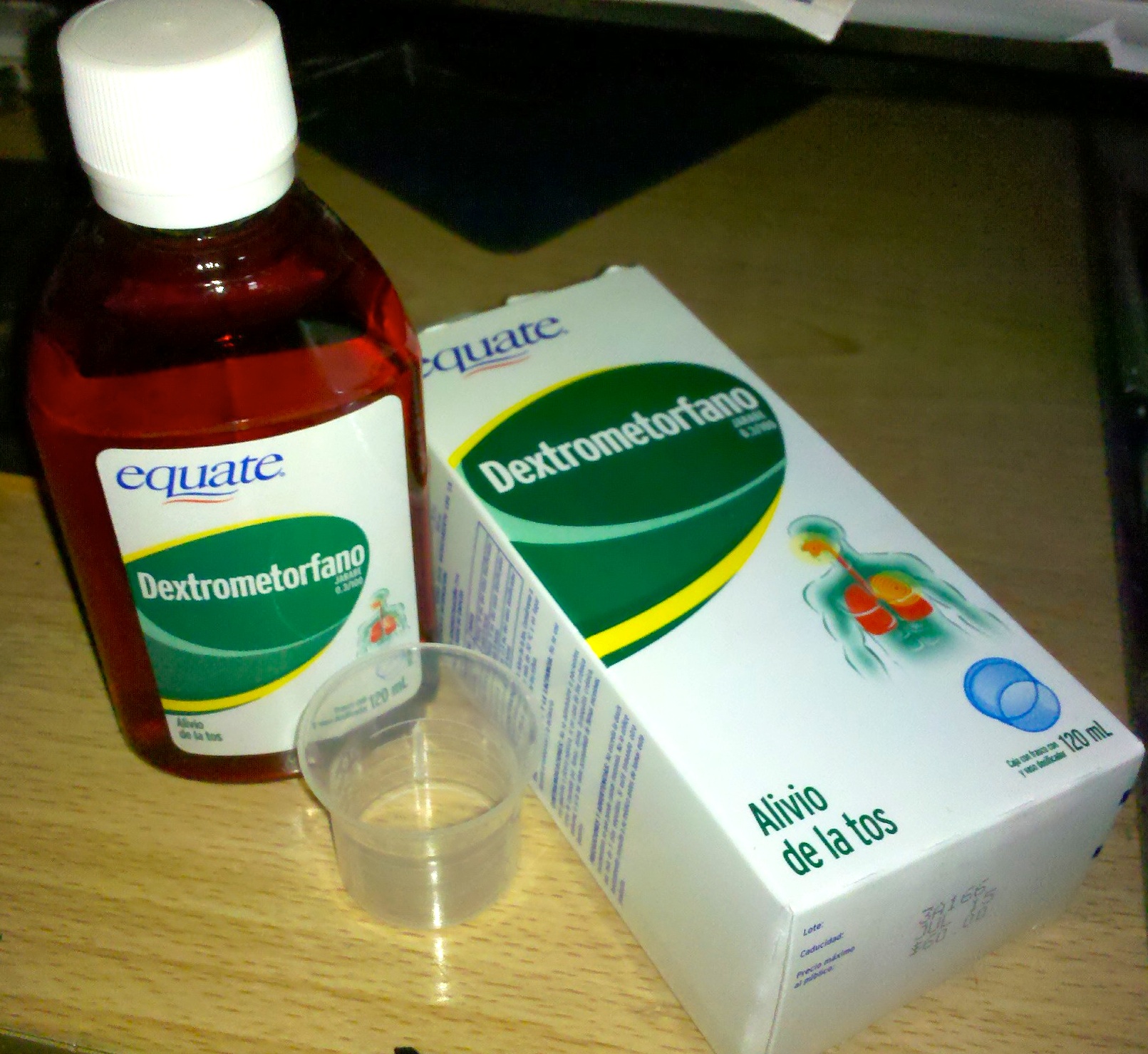
Dextromethorphan
Dextromethorphan (DXM) is a medication most often used as a cough suppressant in over-the-counter cold and cough medicines. It is sold in syrup, tablet, spray, and lozenge forms. In 2022, the FDA approved a formulation of it combined with bupropion named Auvelity to serve as a rapid acting antidepressant in patients with major depressive disorder. It is in the morphinan class of medications with sedative, dissociative, and stimulant properties (at lower doses). Dextromethorphan does not have a significant affinity for the mu-opioid receptor activity typical of morphinan compounds and exerts its therapeutic effects through several other receptors. In its pure form, dextromethorphan occurs as a white powder. Dextromethorphan is also used recreationally. When exceeding approved dosages, dextromethorphan acts as a dissociative hallucinogen. It has multiple mechanisms of action, including actions as a nonselective serotonin reuptake inhibitor and a sigma-1 receptor agonis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theophylline
Theophylline, also known as 1,3-dimethylxanthine, is a phosphodiesterase inhibiting drug used in therapy for respiratory diseases such as chronic obstructive pulmonary disease (COPD) and asthma under a variety of brand names. As a member of the xanthine family, it bears structural and pharmacological similarity to theobromine and caffeine, and is readily found in nature, being present in tea (''Camellia sinensis'') and cocoa (''Theobroma cacao''). A small amount of theophylline is one of the products of caffeine metabolic processing in the liver. Medical uses The main actions of theophylline involve: * relaxing bronchial smooth muscle * increasing heart muscle contractility and efficiency (positive inotrope) * increasing heart rate (positive chronotropic) * increasing blood pressure * increasing renal blood flow * anti-inflammatory effects * central nervous system stimulatory effect mainly on the medullary respiratory center. The main therapeutic uses of theophylline are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section Forms below), hence it is stored as an aqueous solution (formalin), which is also used to store animal specimens. It is the simplest of the aldehydes (). The common name of this substance comes from its similarity and relation to formic acid. Formaldehyde is an important precursor to many other materials and chemical compounds. In 1996, the installed capacity for the production of formaldehyde was estimated at 8.7 million tons per year. It is mainly used in the production of industrial resins, e.g., for particle board and coatings. Forms Formaldehyde is more complicated than many simple carbon compounds in that it adopts several diverse forms. These compounds can often be used interchangeably and can be interconverted. *Molecular for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Cyanoacrylate
Ethyl cyanoacrylate (ECA), a cyanoacrylate ester, is an ethyl ester of 2-cyano- 2-propenoic acid. It is a colorless liquid with low viscosity and a faint sweet smell in pure form. It is the main component of cyanoacrylate glues and can be encountered under many trade names. It is soluble in acetone, methyl ethyl ketone, nitromethane, and methylene chloride. ECA polymerizes rapidly in presence of moisture. Production Ethyl cyanoacrylate is prepared by the condensation of formaldehyde with ethyl cyanoacetate: : + → + This exothermic reaction affords the polymer, which is subsequently sintered, thermally "cracked" to give the monomer. Alternatively, it can be prepared by the ethoxycarbonylation of cyanoacetylene. Applications Ethyl cyanoacrylate is used for gluing various materials. It is also used in medicine, for liquid bandages and for suture-less surgery, but it is used less often than the less toxic ''n''-butyl and octyl cyanoacrylates. Off-the-shelf consum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esterification
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |