|
Cuprates
Cuprate loosely refers to a material that can be viewed as containing anionic copper complexes. Examples include tetrachloridocuprate ( uCl4sup>2−), the superconductor YBa2Cu3O7, and the organocuprates (e.g., dimethylcuprate u(CH3)2sup>−). The term cuprates derives from the Latin word for copper, ''cuprum''. The term is mainly used in three contexts: oxide materials, anionic coordination complexes, and anionic organocopper compounds. Oxides One of the simplest oxide-based cuprates is the copper(III) oxide KCuO2, also known as "potassium cuprate(III)". This species can be viewed as the K+ salt of the polyanion []''n''. As such the material is classified as a cuprate. This dark blue diamagnetic solid is produced by heating potassium peroxide and copper(II) oxide in an atmosphere of oxygen: :K2O2 + 2 CuO → 2 KCuO2 Sodium cuprate(III) NaCuO2 and potassium cuprate(III) KCuO2 can also be produced by using hypochlorites or hypobromites to oxidize copper hydroxide under ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-temperature Superconductivity
High-temperature superconductors (abbreviated high-c or HTS) are defined as materials that behave as superconductors at temperatures above , the boiling point of liquid nitrogen. The adjective "high temperature" is only in respect to previously known superconductors, which function at even colder temperatures close to absolute zero. In absolute terms, these "high temperatures" are still far below ambient, and therefore require cooling. The first high-temperature superconductor was discovered in 1986, by IBM researchers Bednorz and Müller, who were awarded the Nobel Prize in Physics in 1987 "for their important break-through in the discovery of superconductivity in ceramic materials". Most high-c materials are type-II superconductors. The major advantage of high-temperature superconductors is that they can be cooled by using liquid nitrogen, as opposed to the previously known superconductors which require expensive and hard-to-handle coolants, primarily liquid helium. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cuprate Superconductor
Cuprate superconductors are a family of high-temperature superconducting materials made of layers of copper oxides (CuO2) alternating with layers of other metal oxides, which act as charge reservoirs. At ambient pressure, cuprate superconductors are the highest temperature superconductors known. However, the mechanism by which superconductivity occurs is still not understood. History The first cuprate superconductor was found in 1986 in the non-stoichiometric cuprate lanthanum barium copper oxide by IBM researchers Georg Bednorz and Karl Alex Müller. The critical temperature for this material was 35K, well above the previous record of 23 K. The discovery led to a sharp increase in research on the cuprates, resulting in thousands of publications between 1986 and 2001. Bednorz and Müller were awarded the Nobel Prize in Physics in 1987, only a year after their discovery. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium Barium Copper Oxide
Yttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K. Many YBCO compounds have the general formula Y Ba2 Cu3 O7−''x'' (also known as Y123), although materials with other Y:Ba:Cu ratios exist, such as Y Ba2 Cu4 Oy (Y124) or Y2 Ba4 Cu7 Oy (Y247). At present, there is no singularly recognised theory for high-temperature superconductivity. It is part of the more general group of rare-earth barium copper oxides (ReBCO) in which, instead of yttrium, other rare earths are present. History In April 1986, Georg Bednorz and Karl Müller, working at IBM in Zurich, discovered that certain semiconducting oxides became superconducting at relatively high temperature, in particular, a lanthanum barium copper oxide becomes superconducting at 35 K. This oxide was an oxyge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable metallic form ( native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper Compounds
Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called ''cuprous'' and ''cupric'', respectively. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological processes. Binary compounds As with other elements, the simplest compounds of copper are binary compounds, i.e. those containing only two elements, the principal examples being oxides, sulfides, and halides. Both cuprous and cupric oxides are known. Among the numerous copper sulfides, important examples include copper(I) sulfide and copper(II) sulfide. Cuprous halides with fluorine, chlorine, bromine, and iodine are known, as are cupric halides with fluorine, chlorine, and bromine. Attempts to prepare copper(II) iodide yield only copper(I) iodide and iodine. :2 Cu2+ + 4 I− → 2 CuI + I2 Coordination chemistry Copper forms coordination complexes with ligands. In aqueous solution, copper(II) exists as . This compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
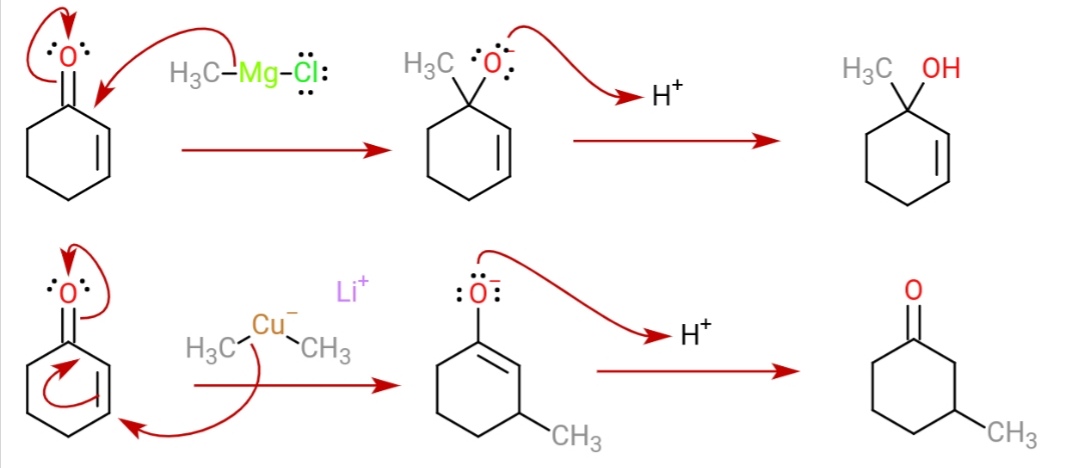
Gilman Reagent
A Gilman reagent is a lithium and copper ( diorganocopper) reagent compound, R2CuLi, where R is an alkyl or aryl. These reagents are useful because, unlike related Grignard reagents and organolithium reagents, they react with organic halides to replace the halide group with an R group (the Corey–House reaction). Such displacement reactions allow for the synthesis of complex products from simple building blocks. Reactions These reagents were discovered by Henry Gilman and coworkers. Lithium dimethylcopper (CH3)2CuLi can be prepared by adding copper(I) iodide to methyllithium in tetrahydrofuran at −78 °C. In the reaction depicted below, the Gilman reagent is a methylating reagent reacting with an alkyne in a conjugate addition, and the negative charge is trapped in a nucleophilic acyl substitution with the ester group forming a cyclic enone. Due to the softness of the nucleophile, they do 1,4 addition on conjugated enones, rather than 1,2 addition. : Struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylating Reagent
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion (carbanion). The formal "alkyl anion" attacks an electrophile, forming a new covalent bond b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidizing Agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an Redox, oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Hexafluorocuprate(IV)
Caesium hexafluorocuprate is the inorganic compound with the chemical formula . It is a red solid that degrades upon contact with water. It was first prepared be heating and caesium fluoride Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electroposit ... at 410°C under 350 atmospheres of fluorine: :2 CsCuCl3 + 2 CsF + 5 F2 → 2 Cs2CuF6 + 3 Cl2 The anion uF6sup>2- is a rare example of a copper(IV) complex. In terms of its electronic structure, the anion has a low-spin d7 configuration. It is thus susceptible to Jahn-Teller distortion. Further reading * * References Caesium compounds Copper compounds Fluoro complexes Metal halides Fluorometallates {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |