|
Cuprate
Cuprate loosely refers to a material that can be viewed as containing anionic copper complexes. Examples include tetrachloridocuprate ( uCl4sup>2−), the superconductor YBa2Cu3O7, and the organocuprates (e.g., dimethylcuprate u(CH3)2sup>−). The term cuprates derives from the Latin word for copper, ''cuprum''. The term is mainly used in three contexts: oxide materials, anionic coordination complexes, and anionic organocopper compounds. Oxides One of the simplest oxide-based cuprates is the copper(III) oxide KCuO2, also known as "potassium cuprate(III)". This species can be viewed as the K+ salt of the polyanion []''n''. As such the material is classified as a cuprate. This dark blue diamagnetic solid is produced by heating potassium peroxide and copper(II) oxide in an atmosphere of oxygen: :K2O2 + 2 CuO → 2 KCuO2 Sodium cuprate(III) NaCuO2 and potassium cuprate(III) KCuO2 can also be produced by using hypochlorites or hypobromites to oxidize copper hydroxide unde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-temperature Superconductivity
High-temperature superconductors (abbreviated high-c or HTS) are defined as materials that behave as superconductors at temperatures above , the boiling point of liquid nitrogen. The adjective "high temperature" is only in respect to previously known superconductors, which function at even colder temperatures close to absolute zero. In absolute terms, these "high temperatures" are still far below ambient, and therefore require cooling. The first high-temperature superconductor was discovered in 1986, by IBM researchers Bednorz and Müller, who were awarded the Nobel Prize in Physics in 1987 "for their important break-through in the discovery of superconductivity in ceramic materials". Most high-c materials are type-II superconductors. The major advantage of high-temperature superconductors is that they can be cooled by using liquid nitrogen, as opposed to the previously known superconductors which require expensive and hard-to-handle coolants, primarily liquid helium. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gilman Reagent
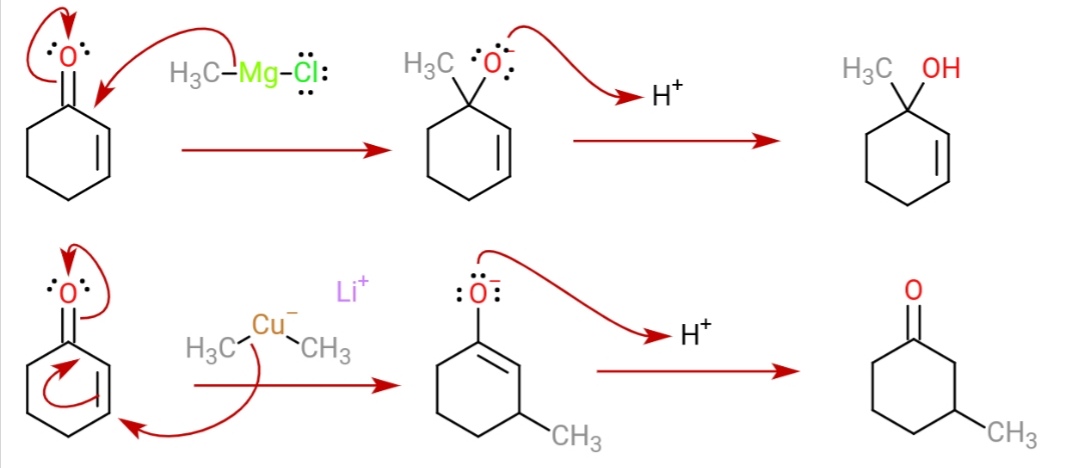
A Gilman reagent is a lithium and copper ( diorganocopper) reagent compound, R2CuLi, where R is an alkyl or aryl. These reagents are useful because, unlike related Grignard reagents and organolithium reagents, they react with organic halides to replace the halide group with an R group (the Corey–House reaction). Such displacement reactions allow for the synthesis of complex products from simple building blocks. Reactions These reagents were discovered by Henry Gilman and coworkers. Lithium dimethylcopper (CH3)2CuLi can be prepared by adding copper(I) iodide to methyllithium in tetrahydrofuran at −78 °C. In the reaction depicted below, the Gilman reagent is a methylating reagent reacting with an alkyne in a conjugate addition, and the negative charge is trapped in a nucleophilic acyl substitution with the ester group forming a cyclic enone. Due to the softness of the nucleophile, they do 1,4 addition on conjugated enones, rather than 1,2 addition. : Struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cuprate Superconductor
Cuprate superconductors are a family of high-temperature superconducting materials made of layers of copper oxides (CuO2) alternating with layers of other metal oxides, which act as charge reservoirs. At ambient pressure, cuprate superconductors are the highest temperature superconductors known. However, the mechanism by which superconductivity occurs is still not understood. History The first cuprate superconductor was found in 1986 in the non-stoichiometric cuprate lanthanum barium copper oxide by IBM researchers Georg Bednorz and Karl Alex Müller. The critical temperature for this material was 35K, well above the previous record of 23 K. The discovery led to a sharp increase in research on the cuprates, resulting in thousands of publications between 1986 and 2001. Bednorz and Müller were awarded the Nobel Prize in Physics in 1987, only a year after their discovery. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organocopper Compound
Organocopper compounds is the chemistry of organometallic compounds containing a carbon to copper chemical bond. Organocopper chemistry is the study of organocopper compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry. The first organocopper compound, the explosive copper(I) acetylide Cu2C2 (Cu−C≡C−Cu), was synthesized by Rudolf Christian Böttger in 1859 by passing acetylene gas through a solution of copper(I) chloride: :C2H2 + 2 CuCl → Cu2C2 + 2 HCl Structure and bonding Organocopper compounds are diverse in structure and reactivity, but organocopper compounds are largely limited in oxidation states to copper(I), sometimes denoted Cu+. As a d10 metal center, it is related to Ni(0), but owing to its higher oxidation state, it engages in less pi-backbonding. Organic derivatives of Cu(II) and Cu(III) are invoked as intermediates but rarely isolated or even observed. In terms of geometry, copper(I) adopts symmetri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable metallic form ( native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper Compounds
Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called ''cuprous'' and ''cupric'', respectively. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological processes. Binary compounds As with other elements, the simplest compounds of copper are binary compounds, i.e. those containing only two elements, the principal examples being oxides, sulfides, and halides. Both cuprous and cupric oxides are known. Among the numerous copper sulfides, important examples include copper(I) sulfide and copper(II) sulfide. Cuprous halides with fluorine, chlorine, bromine, and iodine are known, as are cupric halides with fluorine, chlorine, and bromine. Attempts to prepare copper(II) iodide yield only copper(I) iodide and iodine. :2 Cu2+ + 4 I− → 2 CuI + I2 Coordination chemistry Copper forms coordination complexes with ligands. In aqueous solution, copper(II) exists as . This compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Hexafluorocuprate(IV)
Caesium hexafluorocuprate is the inorganic compound with the chemical formula . It is a red solid that degrades upon contact with water. It was first prepared be heating and caesium fluoride Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electroposit ... at 410°C under 350 atmospheres of fluorine: :2 CsCuCl3 + 2 CsF + 5 F2 → 2 Cs2CuF6 + 3 Cl2 The anion uF6sup>2- is a rare example of a copper(IV) complex. In terms of its electronic structure, the anion has a low-spin d7 configuration. It is thus susceptible to Jahn-Teller distortion. Further reading * * References Caesium compounds Copper compounds Fluoro complexes Metal halides Fluorometallates {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Hexafluorocuprate(III)
Potassium hexafluorocuprate(III) is an inorganic compound with the chemical formula K3CuF6. It is a green paramagnetic solid, a relatively rare example of a copper(III) compound. Synthesis and structure The compound is prepared by oxidizing the mixture of potassium chloride and cuprous chloride with fluorine: :3 KCl + CuCl + 3 F2 → K3CuF6 + 2 Cl2 A variety of analogues are known.R. Hoppe, G. Wingefeld "Zur Kenntnis der Hexafluorocuprate(III)" Zeitschrift für anorganische und allgemeine Chemie 1984, Vol. 519, pages 195–203. The compound reacts with water easily, producing oxygen and copper(II) products. See also *Cuprate(III) Cuprate loosely refers to a material that can be viewed as containing anionic copper complexes. Examples include tetrachloridocuprate ( uCl4sup>2−), the superconductor YBa2Cu3O7, and the organocuprates (e.g., dimethylcuprate u(CH3)2sup>� ... * caesium hexafluorocuprate(IV) References {{Potassium compounds Copper compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium Barium Copper Oxide
Yttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K. Many YBCO compounds have the general formula Y Ba2 Cu3 O7−''x'' (also known as Y123), although materials with other Y:Ba:Cu ratios exist, such as Y Ba2 Cu4 Oy (Y124) or Y2 Ba4 Cu7 Oy (Y247). At present, there is no singularly recognised theory for high-temperature superconductivity. It is part of the more general group of rare-earth barium copper oxides (ReBCO) in which, instead of yttrium, other rare earths are present. History In April 1986, Georg Bednorz and Karl Müller, working at IBM in Zurich, discovered that certain semiconducting oxides became superconducting at relatively high temperature, in particular, a lanthanum barium copper oxide becomes superconducting at 35 K. This oxide was an oxyge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Oxide
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds. Production It is produced on a large scale by pyrometallurgy, as one stage in extracting copper from its ores. The ores are treated with an aqueous mixture of ammonium carbonate, ammonia, and oxygen to give copper(I) and copper(II) ammine complexes, which are extracted from the solids. These complexes are decomposed with steam to give CuO. It can be formed by heating copper in air at around 300–800°C: : 2 Cu + O2 → 2 CuO For laboratory uses, pure copper(II) oxide is better prepared by heating copper(II) nitrate, copper(II) hydroxide, or basic copper(II) carbonate: : 2 Cu(NO3)2(s) → 2 CuO(s) + 4 NO2(g) + O2(g) (180° ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |