|
Cuneane
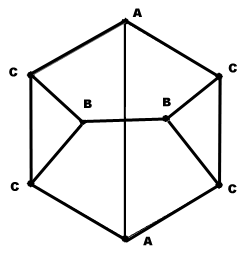
Cuneane (C8H8, pentacyclo .3.0.02,4.03,7.06,8ctane) is a saturated hydrocarbon. Its name is derived from the Latin ''cuneus'', meaning a wedge. Cuneane may be produced from cubane by metal-ion-catalyzed σ-bond rearrangement. Similar reactions are known for homocubane (C9H10) and bishomocubane (C10H12). : Molecular geometry The carbon atoms in the cuneane molecule form a hexahedron with point group C2v. The cuneane molecule has three groups of equivalent carbon atoms (A, B, C), which have also been confirmed by NMR. The molecular graph of the carbon skeleton of cuneane is a regular graph with non-equivalent groups of vertices, and so it is a very important test object for different algorithms of mathematical chemistry. : Derivatives Some cuneane derivatives have liquid crystal Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal may flow like a liquid, but it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cuneane
Cuneane (C8H8, pentacyclo .3.0.02,4.03,7.06,8ctane) is a saturated hydrocarbon. Its name is derived from the Latin ''cuneus'', meaning a wedge. Cuneane may be produced from cubane by metal-ion-catalyzed σ-bond rearrangement. Similar reactions are known for homocubane (C9H10) and bishomocubane (C10H12). : Molecular geometry The carbon atoms in the cuneane molecule form a hexahedron with point group C2v. The cuneane molecule has three groups of equivalent carbon atoms (A, B, C), which have also been confirmed by NMR. The molecular graph of the carbon skeleton of cuneane is a regular graph with non-equivalent groups of vertices, and so it is a very important test object for different algorithms of mathematical chemistry. : Derivatives Some cuneane derivatives have liquid crystal Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal may flow like a liquid, but it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubane
Cubane () is a synthetic hydrocarbon compound that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Before this work, Eaton believed that cubane would be impossible to synthesize due to the "required 90 degree bond angles". The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strained as compared to the 109.45° angle of a tetrahedral carbon. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry. Having high potential energy but kinetic stability makes cubane and its derivative compounds useful for controlled energy storage. For example, octanitrocubane and heptanitrocubane have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated And Unsaturated Compounds
In chemistry, a saturated compound is a chemical compound (or ion) that resists the addition reactions, such as hydrogenation, oxidative addition, and binding of a Lewis acids and bases, Lewis base. The term is used in many contexts and for many classes of chemical compounds. Overall, saturated compounds are less reactive than unsaturated compounds. Saturation is derived from the Latin word ''saturare'', meaning 'to fill'. Organic chemistry Unsaturated compounds generally carry out typical addition reactions that are not possible with saturated compounds such as alkanes. A saturated organic compound has only single bonds between carbon atoms. An important class of saturated compounds are the alkanes. Many saturated compounds have functional groups, e.g., alcohols. Unsaturated organic compounds The concept of saturation can be described using various naming systems, formulas, and Analytical chemistry, analytical tests. For instance, IUPAC nomenclature of organic chemistry, IU ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycyclic Nonaromatic Hydrocarbons
Polycyclic may refer to: * Polycyclic compound, a cyclic compound with more than one hydrocarbon loop or ring structures, including: ** Polycyclic musks ** Polycyclic aromatic hydrocarbon *** Chlorinated polycyclic aromatic hydrocarbon *** Contorted polycyclic aromatic hydrocarbon * Polycyclic group In mathematics, a polycyclic group is a solvable group that satisfies the maximal condition on subgroups (that is, every subgroup is finitely generated). Polycyclic groups are finitely presented, which makes them interesting from a computational ..., in mathematics, a solvable group that satisfies the maximal condition on subgroups * Polycyclic spawning, when an animal reproduces multiple times during its lifespan {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Crystal
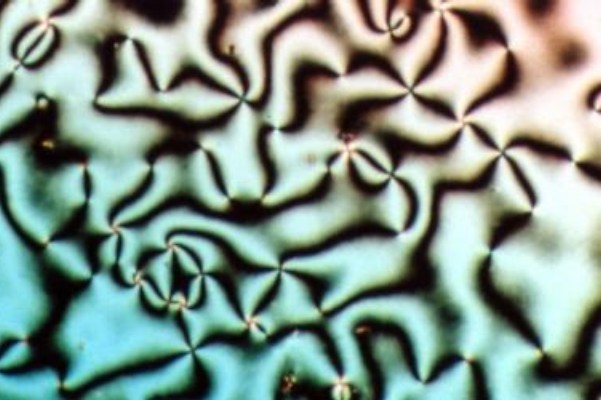
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. LC materials may not always be in a LC state of matter (just as water may be ice or water vapor). Liquid crystals can be divided into 3 main types: * thermotropic, *lyotropic, and * metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions as a function of b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mathematical Chemistry
Mathematical chemistry is the area of research engaged in novel applications of mathematics to chemistry; it concerns itself principally with the mathematical modeling of chemical phenomena. Mathematical chemistry has also sometimes been called computer chemistry, but should not be confused with computational chemistry. Major areas of research in mathematical chemistry include chemical graph theory, which deals with topology such as the mathematical study of isomerism and the development of topological descriptors or indices which find application in quantitative structure-property relationships; and chemical aspects of group theory, which finds applications in stereochemistry and quantum chemistry. Another important area is molecular knot theory and circuit topology that describe the topology of folded linear molecules such as proteins and Nucleic Acids. The history of the approach may be traced back to the 19th century. Georg Helm published a treatise titled "The Principles of M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regular Graph
In graph theory, a regular graph is a graph where each vertex has the same number of neighbors; i.e. every vertex has the same degree or valency. A regular directed graph must also satisfy the stronger condition that the indegree and outdegree of each vertex are equal to each other. A regular graph with vertices of degree is called a graph or regular graph of degree . Also, from the handshaking lemma, a regular graph contains an even number of vertices with odd degree. Regular graphs of degree at most 2 are easy to classify: a graph consists of disconnected vertices, a graph consists of disconnected edges, and a graph consists of a disjoint union of cycles and infinite chains. A graph is known as a cubic graph. A strongly regular graph is a regular graph where every adjacent pair of vertices has the same number of neighbors in common, and every non-adjacent pair of vertices has the same number of neighbors in common. The smallest graphs that are regular but not strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explain many of a molecule's chemical properties, such as whether or not it has a dipole moment, as well as its allowed spectroscopic transitions. To do this it is necessary to use group theory. This involves classifying the states of the molecule using the irreducible representations from the character table of the symmetry group of the molecule. Symmetry is useful in the study of molecular orbitals, with applications to the Hückel method, to ligand field theory, and to the Woodward-Hoffmann rules. Many university level textbooks on physical chemistry, quantum chemistry, spectroscopy and inorganic chemistry discuss symmetry. Another framework on a larger scale is the use of crystal systems to describe crystallographic symmetry in bulk materia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexahedron
A hexahedron (plural: hexahedra or hexahedrons) or sexahedron (plural: sexahedra or sexahedrons) is any polyhedron with six faces. A cube, for example, is a regular hexahedron with all its faces square, and three squares around each vertex. There are seven topologically distinct ''convex'' hexahedra, one of which exists in two mirror image forms. There are three topologically distinct concave hexahedra. Two polyhedra are "topologically distinct" if they have intrinsically different arrangements of faces and vertices, such that it is impossible to distort one into the other simply by changing the lengths of edges or the angles between edges or faces. Convex, Cuboid Convex, Others Concave There are three further topologically distinct hexahedra that can only be realised as ''concave'' figures: A digonal antiprism can be considered a degenerate form of hexahedron, having two opposing digonal faces and four triangular faces. However, digons are usually disregarded in the defi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemische Berichte
''Chemische Berichte'' (usually abbreviated as ''Ber.'' or ''Chem. Ber.'') was a German-language scientific journal of all disciplines of chemistry founded in 1868. It was one of the oldest scientific journals in chemistry, until it merged with ''Recueil des Travaux Chimiques des Pays-Bas'' to form ''Chemische Berichte/Recueil'' in 1997. ''Chemische Berichte/Recueil'' was then merged with other European journals in 1998 to form ''European Journal of Inorganic Chemistry''. History Founded in 1868 as ''Berichte der Deutschen Chemischen Gesellschaft'' (, CODEN BDCGAS), it operated under this title until 1928 (Vol. 61). The journal was then split into: * ''Berichte der Deutschen Chemischen Gesellschaft, A: Vereins-Nachrichten'' (, CODEN BDCAAS), and * ''Berichte der Deutschen Chemischen Gesellschaft, B: Abhandlungen'' (, CODEN BDCBAD). Vol. 78 and 79 (1945–1946) were omitted and not published due to World War II. The journal was renamed ''Chemische Berichte'' (, CODEN CHBEAM) in 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline and lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to the naturally occurring petroleum, natural gas and coal, and to their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are carbon di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |