|
Cottrell Atmosphere
In materials science, the concept of the Cottrell atmosphere was introduced by A. H. Cottrell and B. A. Bilby in 1949 to explain how dislocations are pinned in some metals by boron, carbon, or nitrogen interstitials. Cottrell atmospheres occur in body-centered cubic (BCC) and face-centered cubic (FCC) materials, such as iron or nickel, with small impurity atoms, such as boron, carbon, or nitrogen. As these interstitial atoms distort the lattice slightly, there will be an associated residual stress field surrounding the interstitial. This stress field can be relaxed by the interstitial atom diffusing towards a dislocation, which contains a small gap at its core (as it is a more open structure), see Figure 1. Once the atom has diffused into the dislocation core the atom will stay. Typically only one interstitial atom is required per lattice plane of the dislocation. The collection of solute atoms around the dislocation core due to this process is the Cottrell atmosphere. Influ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stress–strain Curve
In engineering and materials science, a stress–strain curve for a material gives the relationship between stress and strain. It is obtained by gradually applying load to a test coupon and measuring the deformation, from which the stress and strain can be determined (see tensile testing). These curves reveal many of the properties of a material, such as the Young's modulus, the yield strength and the ultimate tensile strength. Definition Generally speaking, curves representing the relationship between stress and strain in any form of deformation can be regarded as stress–strain curves. The stress and strain can be normal, shear, or mixture, also can be uniaxial, biaxial, or multiaxial, even change with time. The form of deformation can be compression, stretching, torsion, rotation, and so on. If not mentioned otherwise, stress–strain curve refers to the relationship between axial normal stress and axial normal strain of materials measured in a tension test. Engineer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscous Drag
In fluid dynamics, drag (sometimes called air resistance, a type of friction, or fluid resistance, another type of friction or fluid friction) is a force acting opposite to the relative motion of any object moving with respect to a surrounding fluid. This can exist between two fluid layers (or surfaces) or between a fluid and a solid surface. Unlike other resistive forces, such as dry friction, which are nearly independent of velocity, the drag force depends on velocity. Drag force is proportional to the velocity for low-speed flow and the squared velocity for high speed flow, where the distinction between low and high speed is measured by the Reynolds number. Even though the ultimate cause of drag is viscous friction, turbulent drag is independent of viscosity. Drag forces always tend to decrease fluid velocity relative to the solid object in the fluid's path. Examples Examples of drag include the component of the net aerodynamic or hydrodynamic force acting opposite to the dire ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Creep (deformation)
In materials science, creep (sometimes called cold flow) is the tendency of a solid material to move slowly or deform permanently under the influence of persistent mechanical stresses. It can occur as a result of long-term exposure to high levels of stress that are still below the yield strength of the material. Creep is more severe in materials that are subjected to heat for long periods and generally increases as they near their melting point. The rate of deformation is a function of the material's properties, exposure time, exposure temperature and the applied structural load. Depending on the magnitude of the applied stress and its duration, the deformation may become so large that a component can no longer perform its function – for example creep of a turbine blade could cause the blade to contact the casing, resulting in the failure of the blade. Creep is usually of concern to engineers and metallurgists when evaluating components that operate under high stresses or hig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homologous Temperature
Homologous temperature expresses the thermodynamic temperature of a material as a fraction of the thermodynamic temperature of its melting point (i.e. using the Kelvin scale): T_H = \frac For example, the homologous temperature of lead at room temperature (25 °C) is approximately 0.50 (TH = T/Tmp = 298 K/601 K = 0.50). Significance of the homologous temperature The homologous temperature of a substance is useful for determining the rate of steady state creep (diffusion dependent deformation). A higher homologous temperature results in an exponentially higher rate of diffusion dependent deformation. Additionally, for a given fixed homologous temperature, two materials with different melting points would have similar diffusion-dependent deformation behaviour. For example, solder (Tmp = 456 K) at 115 °C would have comparable mechanical properties to copper (Tmp = 1358 K) at 881 °C, because they would both be at 0.85Tmp despite being at diff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine. Titanium was discovered in Cornwall, Great Britain, by William Gregor in 1791 and was named by Martin Heinrich Klaproth after the Titans of Greek mythology. The element occurs within a number of minerals, principally rutile and ilmenite, which are widely distributed in the Earth's crust and lithosphere; it is found in almost all living things, as well as bodies of water, rocks, and soils. The metal is extracted from its principal mineral ores by the Kroll and Hunter processes. The most common compound, titanium dioxide, is a popular photocatalyst and is used in the manufacture of white pigments. Other compounds include titanium tetrachloride (TiCl4), a component of smoke screens and catalysts; and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarburizing
Decarburization (or decarbonization) is the process of decreasing carbon content, which is the opposite of carburization. The term is typically used in metallurgy, describing the decrease of the content of carbon in metals (usually steel). Decarburization occurs when the metal is heated to temperatures of 700 °C or above when carbon in the metal reacts with gases containing oxygen or hydrogen. The removal of carbon removes hard carbide phases resulting in a softening of the metal, primarily at the surfaces which are in contact with the decarburizing gas. Decarburization can be either advantageous or detrimental, depending on the application for which the metal will be used. It is thus both something that can be done intentionally as a step in a manufacturing process, or something that happens as a side effect of a process (such as rolling) and must be either prevented or later reversed (such as via a carburization step). The decarburization mechanism can be described as th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interstitial Free Steel
An interstitial space or interstice is a space between structures or objects. In particular, interstitial may refer to: Biology * Interstitial cell tumor * Interstitial cell, any cell that lies between other cells * Interstitial collagenase, enzyme that breaks the peptide bonds in collagen * Interstitial cystitis * Interstitium, the contiguous fluid-filled space existing between the skin and body organs * Interstitial fluid, a solution that bathes and surrounds the cells of multicellular animals * Interstitial granulomatous dermatitis * Interstitial infusion * Interstitial keratitis * Interstitial lung disease * Interstitial nephritis * Interstitial pregnancy Other uses To describe the spaces within particulate matter such sands, gravels, cobbles, grain, etc. that lie between the discrete particles. * Interstitial art * Interstitial condensation, in construction * Interstitial site, in chemistry * Interstitial defect, in chemistry * Interstitial television show, in televis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lüders Band
Lüders bands, is type of slip bands in metals or stretcher-strain marks which are formed due to localized bands of plastic deformation in metals experiencing tensile stresses, common to low-carbon steels and certain Al-Mg alloys. First reported by Guillaume Piobert, and later by W. Lüders, the mechanism that stimulates their appearance is known as dynamic strain aging, or the inhibition of dislocation motion by interstitial atoms (in steels, typically carbon and nitrogen), around which "atmospheres" or "zones" naturally congregate. As internal stresses tend to be highest at the shoulders of tensile test specimens, band formation is favored in those areas. However, the formation of Lüders bands depends primarily on the microscopic (i.e. average grain size and crystal structure, if applicable) and macroscopic geometries of the material. For example, a tensile-tested steel bar with a square cross-section tends to develop comparatively more bands than would a bar of identical com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Room Temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on humidity, air circulation and other factors. Food or beverages may be served at ''room temperature'', meaning neither heated nor cooled. In certain fields, like science and engineering, and within a particular context, ''room temperature'' can mean different agreed-upon ranges. In contrast, ''ambient temperature'' is the actual temperature, as measured by a thermometer, of the air (or other medium and surroundings) in any particular place. The ambient temperature (e.g. an unheated room in winter) may be very different from an ideal ''room temperature''. Comfort temperatures ''The American Heritage Dictionary of the English Language'' identifies room temperature as around , while the ''Oxford English Dictionary'' states that it is "conv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
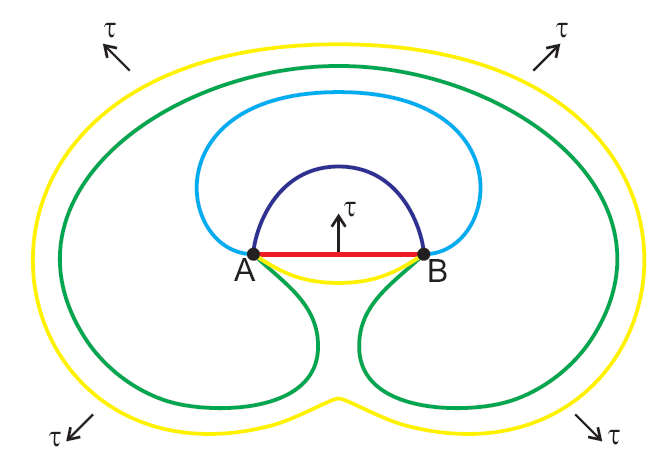
Frank–Read Source
In materials science, a Frank–Read source is a mechanism explaining the generation of multiple dislocations in specific well-spaced slip planes in crystals when they are deformed. When a crystal is deformed, in order for slip to occur, dislocations must be generated in the material. This implies that, during deformation, dislocations must be primarily generated in these planes. Cold working of metal increases the number of dislocations by the Frank–Read mechanism. Higher dislocation density increases yield strength and causes work hardening of metals. The mechanism of dislocation generation was proposed by and named after British physicist Charles Frank and Thornton Read. History Charles Frank detailed the history of the discovery from his perspective in ''Proceedings of the Royal Society'' in 1980. In 1950 Charles Frank, who was then a research fellow in the physics department at the University of Bristol, visited the United States to participate in a conference on cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |