|
Constantino Tsallis
Constantino Tsallis (; el, Κωνσταντίνος Τσάλλης ; born 4 November 1943) is a naturalized Brazilian physicist of Greek descent, working in Rio de Janeiro at Centro Brasileiro de Pesquisas Físicas (CBPF), Brazil. Biography Tsallis was born in Greece, and grew up in Argentina, where he studied physics at Instituto Balseiro, in Bariloche. In 1974, he received a ''Doctorat d'État ès Sciences Physiques'' degree from the University of Paris-Sud. He moved to Brazil in 1975 with his wife and daughter. Tsallis is an External Professor of the Santa Fe Institute. In 2011 he gave a talk ''From Nonlinear Statistical Mechanics to Nonlinear Quantum Mechanics — Concepts and Applications'' at the international symposium on subnuclear physics held in Vatican City. Research Tsallis is credited with introducing the notion of what is known as Tsallis entropy and Tsallis statistics in his 1988 paper "Possible generalization of Boltzmann–Gibbs statistics" published i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Athens
Athens ( ; el, Αθήνα, Athína ; grc, Ἀθῆναι, Athênai (pl.) ) is both the capital city, capital and List of cities and towns in Greece, largest city of Greece. With a population close to four million, it is also the seventh List of urban areas in the European Union, largest city in the European Union. Athens dominates and is the capital of the Attica (region), Attica region and is one of the List of oldest continuously inhabited cities, world's oldest cities, with its recorded history spanning over 3,400 years and its earliest human presence beginning somewhere between the 11th and 7th millennia BC. Classical Athens was a powerful Greek city-state, city-state. It was a centre for the arts, learning and philosophy, and the home of Plato's Platonic Academy, Academy and Aristotle's Lyceum (classical), Lyceum. It is widely referred to as the cradle of civilization, cradle of Western culture, Western civilization and the democracy#History, birthplace of democracy, larg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Instituto Balseiro
Balseiro Institute ( es, Instituto Balseiro) is an academic institution that belongs partially to the National University of Cuyo and partially to Argentina's National Atomic Energy Commission. It is located in Bariloche, Río Negro province, Argentina. Notable alumni of this institute include Marcela Carena, Juan Maldacena, Juan Ignacio Galvan and Jorge Pullin. Overview The Balseiro Institute teaches Physics, Nuclear Engineering, Mechanical Engineering and Telecommunications Engineering at undergraduate and graduate levels. The institute admits students who have completed two years of university studies (either in Physics or Engineering) and undergoes a rigorous admission procedure. It's considered the best Experimental Physics and Nuclear Engineering study centre of Latin America, as well as a very prestigious one worldwide. This is a free public institution with a number of features that make it unique. It was created in 1955 and it was formalized in the agreement signed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistical Mechanics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. It does not assume or postulate any natural laws, but explains the macroscopic behavior of nature from the behavior of such ensembles. Statistical mechanics arose out of the development of classical thermodynamics, a field for which it was successful in explaining macroscopic physical properties—such as temperature, pressure, and heat capacity—in terms of microscopic parameters that fluctuate about average values and are characterized by probability distributions. This established the fields of statistical thermodynamics and statistical physics. The founding of the field of statistical mechanics is generally credited to three physicists: *Ludwig Boltzmann, who developed the fundamental interpretation of entropy in terms of a collection of microstates *James Clerk Maxwell, who developed models of probability ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hamiltonian System
A Hamiltonian system is a dynamical system governed by Hamilton's equations. In physics, this dynamical system describes the evolution of a physical system such as a planetary system or an electron in an electromagnetic field. These systems can be studied in both Hamiltonian mechanics and dynamical systems theory. Overview Informally, a Hamiltonian system is a mathematical formalism developed by Hamilton to describe the evolution equations of a physical system. The advantage of this description is that it gives important insights into the dynamics, even if the initial value problem cannot be solved analytically. One example is the planetary movement of three bodies: while there is no closed-form solution to the general problem, Poincaré showed for the first time that it exhibits deterministic chaos. Formally, a Hamiltonian system is a dynamical system characterised by the scalar function H(\boldsymbol,\boldsymbol,t), also known as the Hamiltonian. The state of the system ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optical Lattice
An optical lattice is formed by the interference of counter-propagating laser beams, creating a spatially periodic polarization pattern. The resulting periodic potential may trap neutral atoms via the Stark shift. Atoms are cooled and congregate at the potential extrema (at maxima for blue-detuned lattices, and minima for red-detuned lattices). The resulting arrangement of trapped atoms resembles a crystal lattice and can be used for quantum simulation. Atoms trapped in the optical lattice may move due to quantum tunneling, even if the potential well depth of the lattice points exceeds the kinetic energy of the atoms, which is similar to the electrons in a conductor. However, a superfluid– Mott insulator transition may occur, if the interaction energy between the atoms becomes larger than the hopping energy when the well depth is very large. In the Mott insulator phase, atoms will be trapped in the potential minima and cannot move freely, which is similar to the electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
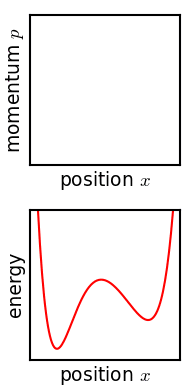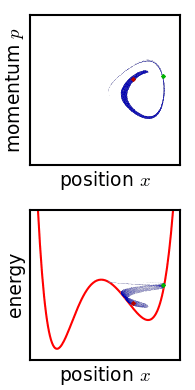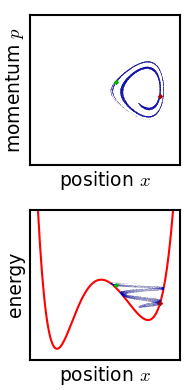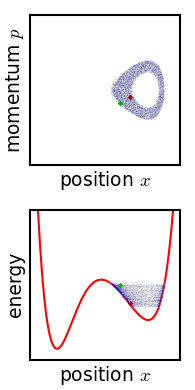
Metastable State
In chemistry and physics, metastability denotes an intermediate energetic state within a dynamical system other than the system's state of least energy. A ball resting in a hollow on a slope is a simple example of metastability. If the ball is only slightly pushed, it will settle back into its hollow, but a stronger push may start the ball rolling down the slope. Bowling pins show similar metastability by either merely wobbling for a moment or tipping over completely. A common example of metastability in science is isomerisation. Higher energy isomers are long lived because they are prevented from rearranging to their preferred ground state by (possibly large) barriers in the potential energy. During a metastable state of finite lifetime, all state-describing parameters reach and hold stationary values. In isolation: *the state of least energy is the only one the system will inhabit for an indefinite length of time, until more external energy is added to the system (unique " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ergodic Theory
Ergodic theory (Greek: ' "work", ' "way") is a branch of mathematics that studies statistical properties of deterministic dynamical systems; it is the study of ergodicity. In this context, statistical properties means properties which are expressed through the behavior of time averages of various functions along trajectories of dynamical systems. The notion of deterministic dynamical systems assumes that the equations determining the dynamics do not contain any random perturbations, noise, etc. Thus, the statistics with which we are concerned are properties of the dynamics. Ergodic theory, like probability theory, is based on general notions of measure theory. Its initial development was motivated by problems of statistical physics. A central concern of ergodic theory is the behavior of a dynamical system when it is allowed to run for a long time. The first result in this direction is the Poincaré recurrence theorem, which claims that almost all points in any subset of the ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann Entropy
In statistical mechanics, Boltzmann's equation (also known as the Boltzmann–Planck equation) is a probability equation relating the entropy S, also written as S_\mathrm, of an ideal gas to the multiplicity (commonly denoted as \Omega or W), the number of real microstates corresponding to the gas's macrostate: where k_\mathrm B is the Boltzmann constant (also written as simply k) and equal to 1.380649 × 10−23 J/K, and \log is the natural logarithm function. In short, the Boltzmann formula shows the relationship between entropy and the number of ways the atoms or molecules of a certain kind of thermodynamic system can be arranged. History The equation was originally formulated by Ludwig Boltzmann between 1872 and 1875, but later put into its current form by Max Planck in about 1900. To quote Planck, "the logarithmic connection between entropy and probability was first stated by L. Boltzmann in his kinetic theory of gases". A 'microstate' is a state spec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intensive And Extensive Properties
Physical properties of materials and systems can often be categorized as being either intensive or extensive, according to how the property changes when the size (or extent) of the system changes. According to IUPAC, an intensive quantity is one whose magnitude is independent of the size of the system, whereas an extensive quantity is one whose magnitude is additive for subsystems. The terms ''intensive and extensive quantities'' were introduced into physics by German writer Georg Helm in 1898, and by American physicist and chemist Richard C. Tolman in 1917. An intensive property does not depend on the system size or the amount of material in the system. It is not necessarily homogeneously distributed in space; it can vary from place to place in a body of matter and radiation. Examples of intensive properties include temperature, ''T''; refractive index, ''n''; density, ''ρ''; and hardness, ''η''. By contrast, extensive properties such as the mass, volume and entropy of sys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Statistical Physics
The ''Journal of Statistical Physics'' is a biweekly publication containing both original and review papers, including book reviews. All areas of statistical physics as well as related fields concerned with collective phenomena in physical systems are covered. The ''Journal of Statistical Physics'' has an impact factor of 1.243 (2019). The journal was established by Howard Reiss. Joel L. Lebowitz is the honorary editor. In the period 1969-1979 the journal published about 65 articles per year, while in the 1980-2016 period approximately 220 articles per year. In total, as to 2017, more than 9000 articles have appeared on this journal. According to Web of Science as of July 2017 the 10 most cited articles which have appeared on this journal are: # Tsallis, C, Possible generalization of Boltzmann-Gibbs statistics, J. Stat. Phys., vol. 52(1-2), 479-487, (1988). Times Cited: 4,245 # Feigenbaum, MJ, Quantitative universality for a class of non-linear transformations, J. Stat. Phys., ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |