|
Complex Metal Hydride
Complex metal hydrides are salts wherein the anions contain hydrides. In the older chemical literature as well as contemporary materials science textbooks, a "metal hydride" is assumed to be nonmolecular, i.e. three-dimensional lattices of atomic ions. In such systems, hydrides are often interstitial and nonstoichiometric, and the bonding between the metal and hydrogen atoms is significantly ionic. In contrast, complex metal hydrides typically contain more than one type of metal or metalloid and may be soluble but invariably react with water. They exhibit ionic bonding between a positive metal ion with molecular anions containing the hydride. In such materials the hydrogen is bonded with significant covalent character to the second metal or metalloid atoms.Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. . Examples In general, complex metal hydrides have the formula MxM'yHn, where M is an alkali metal cation or cation complex and M' is a meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-centered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Borohydride
Lithium borohydride (LiBH4) is a borohydride and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium borohydride, the lithium salt offers some advantages, being a stronger reducing agent and highly soluble in ethers, whilst remaining safer to handle than lithium aluminium hydride.Luca Banfi, Enrica Narisano, Renata Riva, Ellen W. Baxter, "Lithium Borohydride" e-EROS Encyclopedia of Reagents for Organic Synthesis, 2001, John Wiley & Sons. . Preparation Lithium borohydride may be prepared by the metathesis reaction, which occurs upon ball-milling the more commonly available sodium borohydride and lithium bromide: : NaBH4 + LiBr → NaBr + LiBH4 Alternatively, it may be synthesized by treating boron trifluoride with lithium hydride in diethyl ether: : BF3 + 4 LiH → LiBH4 + 3 LiF Reactions Lithium borohydride is a stronger reducing agent than sodium borohydride. In mixtures of methanol and diethyl ether, lithium borohydride is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Hydrides
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-centered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Storage
Hydrogen storage can be accomplished by several existing methods of holding hydrogen for later use. These include mechanical approaches such as using high pressures and low temperatures, or employing chemical compounds that release H2 upon demand. While large amounts of hydrogen are produced by various industries, it is mostly consumed at the site of production, notably for the synthesis of ammonia. For many years hydrogen has been stored as compressed gas or cryogenic liquid, and transported as such in cylinders, tubes, and cryogenic tanks for use in industry or as propellant in space programs. Interest in using hydrogen for on-board storage of energy in zero-emissions vehicles is motivating the development of new methods of storage, more adapted to this new application. The overarching challenge is the very low boiling point of H2: it boils around 20.268 K (−252.882 °C or −423.188 °F). Achieving such low temperatures requires expending significant energy. Es ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-centered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
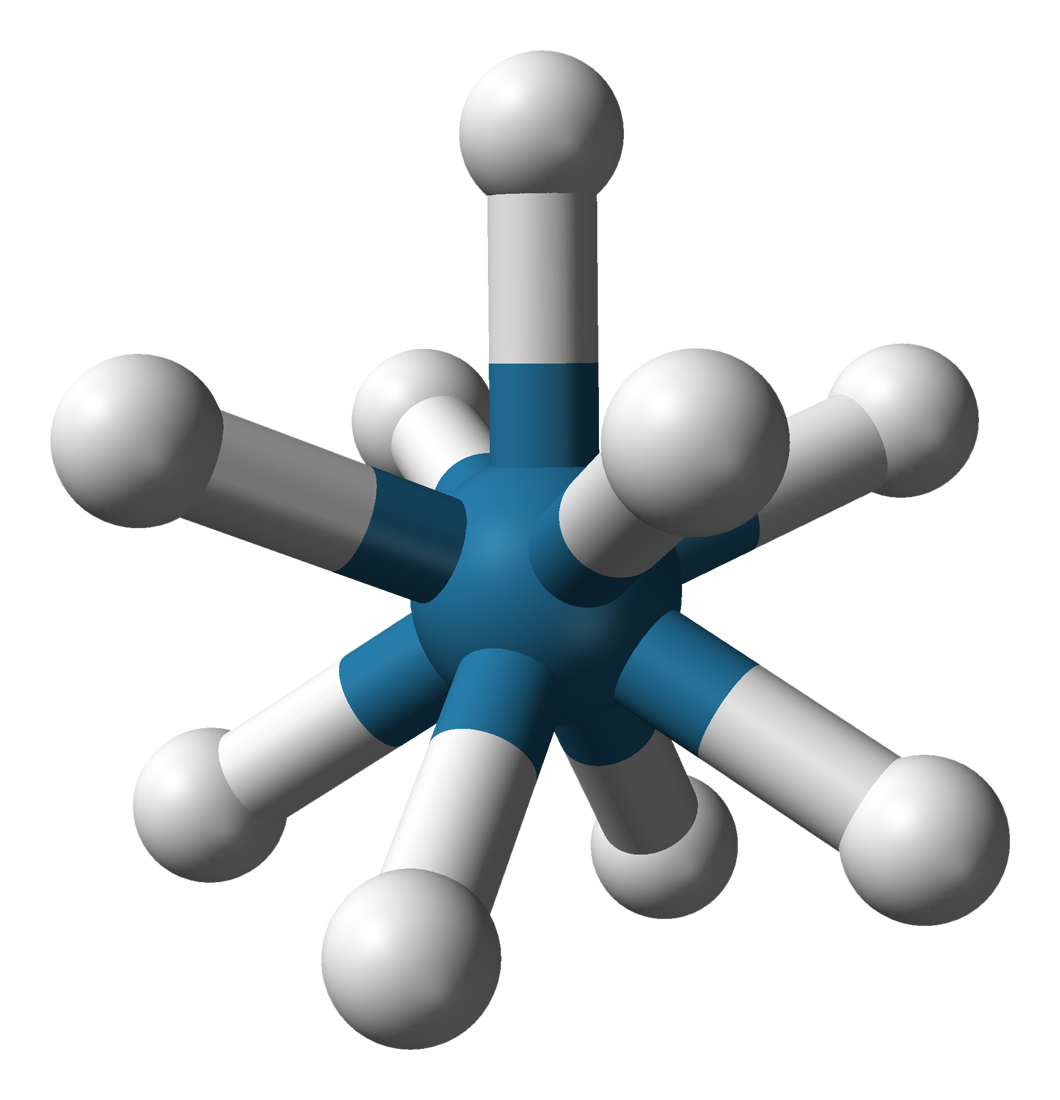
Potassium Nonahydridorhenate
Potassium nonahydridorhenate(VII) is an inorganic compound having the formula K2ReH9. This colourless salt is soluble in water but only poorly soluble in most alcohols. The anion is a rare example of a coordination complex bearing only hydride ligands. History The study of rhenium hydrides can be traced to the 1950s and included reports of the "rhenide" anion, supposedly Re−. These reports led to a series of investigations by A. P. Ginsberg and coworkers on the products from the reduction of perrhenate. The ''rhenide'' anion, Re−, was based on the product of the reduction of perrhenate salts, such as the reduction of potassium perrhenate () by potassium metal. "Potassium rhenide" was shown to exist as a tetrahydrated complex, with the postulated chemical formula . This compound exhibits strongly reducing properties, and slowly yields hydrogen gas when dissolved in water. The lithium and thallous salts were also reported. Later research, however, indicates that the "rhen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent C–O–C linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of oxygen in ethers, alcohols, and water is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Aluminum Hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage. Properties, structure, preparation LAH is a colourless solid but commercial samples are usually gray due to contamination. This material can be purified by recrystallization from diethyl ether. Large-scale purifications employ a Soxhlet extractor. Commonly, the impure gray material is used in synthesis, since the impurities are innocuous and can be easily separated from the organic products. The pure powdered material is pyrophoric, but not its large crystals. Some commercial materials contain mineral oil to inhib ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid State Chemistry
Solid-state chemistry, also sometimes referred as materials chemistry, is the study of the synthesis, structure, and properties of solid phase materials, particularly, but not necessarily exclusively of, non-molecular solids. It therefore has a strong overlap with solid-state physics, mineralogy, crystallography, ceramics, metallurgy, thermodynamics, materials science and electronics with a focus on the synthesis of novel materials and their characterisation. Solids can be classified as crystalline or amorphous on basis of the nature of order present in the arrangement of their constituent particles. History Because of its direct relevance to products of commerce, solid state inorganic chemistry has been strongly driven by technology. Progress in the field has often been fueled by the demands of industry, sometimes in collaboration with academia. Applications discovered in the 20th century include zeolite and platinum-based catalysts for petroleum processing in the 1950s, high-pur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Aluminium Hydride
Sodium aluminium hydride or sodium alanate is an inorganic compound with the chemical formula NaAlH4. It is a white pyrophoric solid that dissolves in tetrahydrofuran (THF), but not in diethyl ether or hydrocarbons. It has been evaluated as an agent for the reversible storage of hydrogen and it is used as a reagent for the chemical synthesis of organic compounds. Similar to lithium aluminium hydride, it is a salt consisting of separated sodium cations and tetrahedral AlH anions. Structure, preparation, and reactions Sodium tetrahydroaluminate adopts the structure of (is isostructural with) calcium tungstate. As such, the tetrahedral AlH centers are linked with eight-coordinat Na+ cations. The compound is prepared from the elements under high pressures of H2 at 200 °C using triethylaluminium catalyst: :Na + Al + 2 H2 → NaAlH4 As a suspension in diethyl ether, it reacts with lithium chloride to give the popular reagent lithium aluminium hydride: :LiCl + NaAl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group 13 Element
The Group 13 network ( pl, Trzynastka, Yiddish: ''דאָס דרײַצענטל'') was a Jewish Nazi collaborationist organization in the Warsaw Ghetto during the German occupation of Poland in World War II. The rise and fall of the Group was likely a proxy for power struggles between various factions in the Nazi German military and bureaucracy, for their own financial benefit. Background The group was founded in December 1940 and led by Abraham Gancwajch, the former head of Hashomer Hatzair in Łódź. ''The Thirteen'' took its informal name from the address of its main office at 13 Leszno Street in Warsaw. Sanctioned by Sicherheitsdienst (SD), and also known as the Jewish Gestapo, the unit reported directly to the local Gestapo office. Organizational structure Group 13 had between 300 and 400 uniformed Jewish officers, distinguished by caps with green bands. Membership in the ''13'' required payment of several thousand zlotys, issued by the German Nazi-controlled bank. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |