|
Cofilin Family
ADF/cofilin is a family of actin-binding proteins associated with the rapid depolymerization of actin microfilaments that give actin its characteristic dynamic instability. This dynamic instability is central to actin's role in muscle contraction, cell motility and transcription regulation. Three highly conserved and highly (70%-82%) identical genes belonging to this family have been described in humans and mice: * CFL1, coding for cofilin 1 (non-muscle, or n-cofilin) * CFL2, coding for cofilin 2 (found in muscle: m-cofilin) * DSTN, coding for destrin, also known as ADF or actin depolymerizing factor Actin-binding proteins regulate assembly and disassembly of actin filaments.Cooper, G. M. and R. E. Hausman. ''The Cell: A Molecular Approach,'' 3rd ed. Washington DC: ASM Press 2004 pp.436-440. Cofilin, a member of the ADF/cofilin family is actually a protein with 70% sequence identity to destrin, making it part of the ADF/cofilin family of small ADP-binding proteins. The pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PDB 1cof EBI
PDB may refer to: * Chess Problem Database Server (PDB Server) * 1,4-Dichlorobenzene (paradichlorobenzene) * Party of German-speaking Belgians, (German: '), a political party and predecessor of the ProDG * PDB (Palm OS), a container format for record databases in Palm OS, Garnet OS and Access Linux Platform * ''Pee Dee Belemnite'', a standard for stable Carbon-13 and Oxygen-18 isotopes; see * Pluggable database, such as an Oracle Database in a multitenancy environment * Potato dextrose broth, a common microbiological growth media * Pousette-Dart Band * President's Daily Brief or Briefing or Bulletin, a top-secret intelligence document produced each morning for the U.S. President * Program database, a file format for storing debugging information * Promised Day Brigade, an Iraqi Shia organisation * Protein Data Bank * Protein Data Bank (file format) The Protein Data Bank (PDB) file format is a textual file format describing the three-dimensional structures of molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arp2/3 Complex
Arp2/3 complex (Actin Related Protein 2/3 complex) is a seven-subunit protein complex that plays a major role in the regulation of the actin cytoskeleton. It is a major component of the actin cytoskeleton and is found in most actin cytoskeleton-containing eukaryotic cells. Two of its subunits, the ''A''ctin-''R''elated ''P''roteins ARP2 and ARP3, closely resemble the structure of monomeric actin and serve as nucleation sites for new actin filaments. The complex binds to the sides of existing ("mother") filaments and initiates growth of a new ("daughter") filament at a distinctive 70 degree angle from the mother. Branched actin networks are created as a result of this nucleation of new filaments. The regulation of rearrangements of the actin cytoskeleton is important for processes like cell locomotion, phagocytosis, and intracellular motility of lipid vesicles. The Arp2/3 complex was named after it was identified in 1994 by affinity chromatography from ''Acanthamoeba castellanii' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
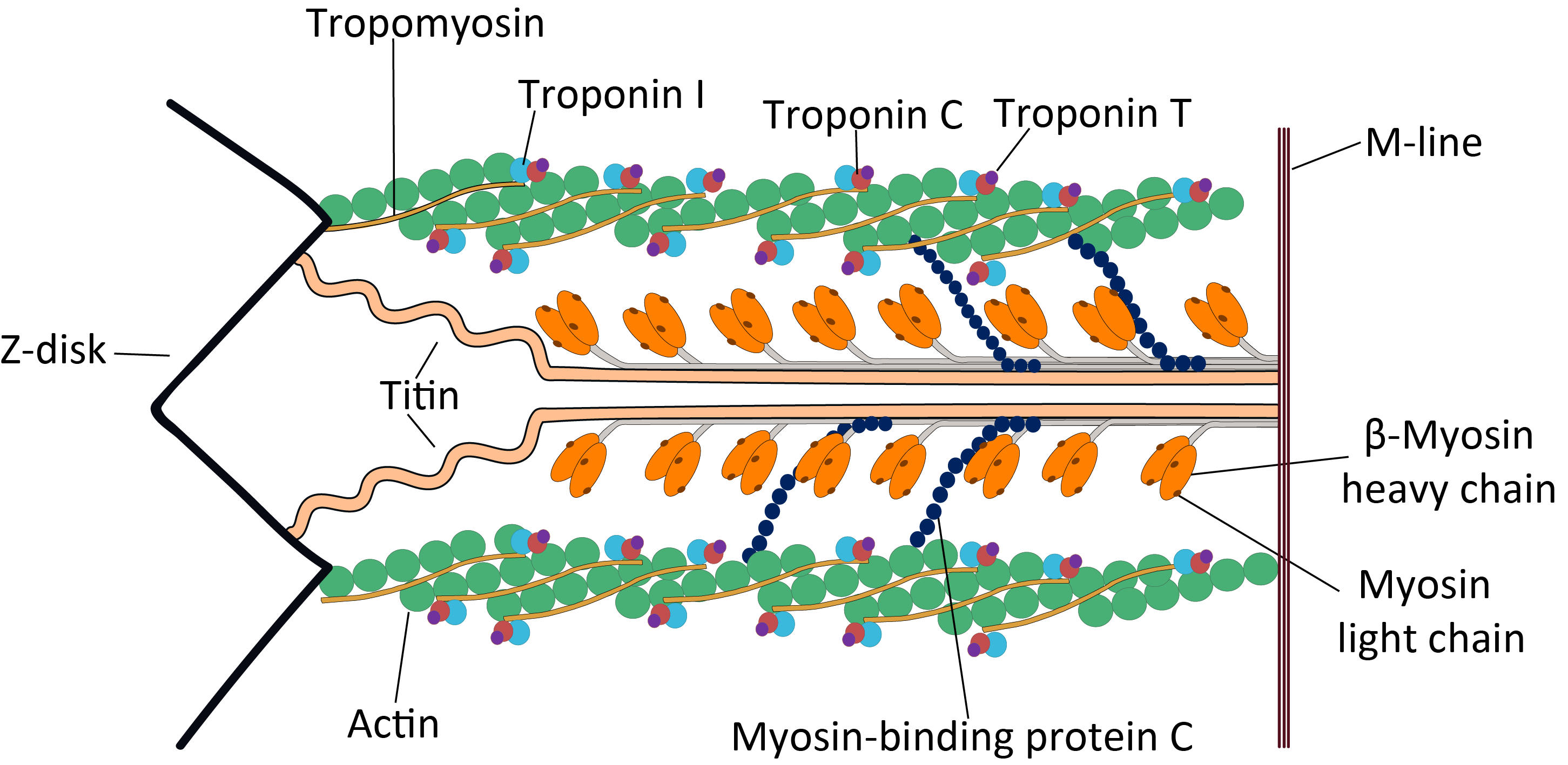
Tropomyosin
Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in actin-based cytoskeletons. Tropomyosin and the actin skeleton All organisms contain organelles that provide physical integrity to their cells. These type of organelles are collectively known as the cytoskeleton, and one of the most ancient systems is based on filamentous polymers of the protein actin. A polymer of a second protein, tropomyosin, is an integral part of most actin filaments in animals. Tropomyosins are a large family of integral components of actin filaments that play a critical role in regulating the function of actin filaments in both muscle and nonmuscle cells. These proteins consist of rod-shaped coiled-coil hetero- or homo- dimers that lie along the α-helical groove of most actin filaments. Interaction occurs along the length of the actin filament, with dimers aligning in a head-to-tail fashion. Tropomyosins are often categorised into two groups, muscle tropomyosin isoforms and nonmus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropomyosin Bound To Actin
Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in actin-based cytoskeletons. Tropomyosin and the actin skeleton All organisms contain organelles that provide physical integrity to their cells. These type of organelles are collectively known as the cytoskeleton, and one of the most ancient systems is based on filamentous polymers of the protein actin. A polymer of a second protein, tropomyosin, is an integral part of most actin filaments in animals. Tropomyosins are a large family of integral components of actin filaments that play a critical role in regulating the function of actin filaments in both muscle and nonmuscle cells. These proteins consist of rod-shaped coiled-coil hetero- or homo-Protein dimer, dimers that lie along the Alpha helix, α-helical groove of most actin filaments. Interaction occurs along the length of the actin filament, with dimers aligning in a head-to-tail fashion. Tropomyosins are often categorised into two groups, muscle tropo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lim Kinase
LIM kinase-1 (LIMK1) and LIM kinase-2 (LIMK2) are actin-binding kinases that phosphorylate members of the ADF/cofilin family of actin binding and filament severing proteins. ADF/cofilin are the only substrates yet identified for the LIM kinases. LIM kinases directly phosphorylate and inactivate members of the cofilin family, resulting in stabilization of filamentous (F)-actin. Lim kinases are activated by signaling through small GTPases of the Rho family. Upstream, LIMK1 is regulated by Pak1, and LIMK2 by the Rho-dependent kinase ROCK. Lim Kinases are activated by PAK (p21-activated kinase). Recent work indicates that LIMK activity is also modulated by HIV-1 viral proteins. There are approximately 40 known eukaryotic LIM proteins, so named for the LIM domains they contain. LIM domains are highly conserved cysteine-rich structures containing 2 zinc fingers. Although zinc fingers usually function by binding to DNA or RNA, the LIM motif probably mediates protein–protein intera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lamellipodia
The lamellipodium (plural lamellipodia) (from Latin ''lamella'', related to ', "thin sheet", and the Greek radical ''pod-'', "foot") is a cytoskeletal protein actin projection on the leading edge of the cell. It contains a quasi-two-dimensional actin mesh; the whole structure propels the cell across a substrate. Within the lamellipodia are ribs of actin called microspikes, which, when they spread beyond the lamellipodium frontier, are called filopodia. The lamellipodium is born of actin nucleation in the plasma membrane of the cell and is the primary area of actin incorporation or microfilament formation of the cell. Description Lamellipodia are found primarily in all mobile cells, such as the keratinocytes of fish and frogs, which are involved in the quick repair of wounds. The lamellipodia of these keratinocytes allow them to move at speeds of 10–20 μm / min over epithelial surfaces. When separated from the main part of a cell, a lamellipodium can still c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenopus Laevis
The African clawed frog (''Xenopus laevis'', also known as the xenopus, African clawed toad, African claw-toed frog or the ''platanna'') is a species of African aquatic frog of the family Pipidae. Its name is derived from the three short claws on each hind foot, which it uses to tear apart its food. The word ''Xenopus'' means 'strange foot' and ''laevis'' means 'smooth'. The species is found throughout much of Sub-Saharan Africa (Nigeria and Sudan to South Africa),Weldon; du Preez; Hyatt; Muller; and Speare (2004). Origin of the Amphibian Chytrid Fungus.' Emerging Infectious Diseases 10(12). and in isolated, introduced populations in North America, South America, Europe, and Asia. All species of the family Pipidae are tongueless, toothless and completely aquatic. They use their hands to shove food in their mouths and down their throats and a hyobranchial pump to draw or suck things in their mouth. Pipidae have powerful legs for swimming and lunging after food. They also use the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gelsolin
Gelsolin is an actin-binding protein that is a key regulator of actin filament assembly and disassembly. Gelsolin is one of the most potent members of the actin-severing gelsolin/villin superfamily, as it severs with nearly 100% efficiency. Cellular gelsolin, found within the cytosol and mitochondria, has a closely related secreted form, Plasma gelsolin, that contains an additional 24 AA N-terminal extension. Plasma gelsolin's ability to sever actin filaments helps the body recover from disease and injury that leaks cellular actin into the blood. Additionally it plays important roles in host innate immunity, activating macrophages and localizing of inflammation. Structure Gelsolin is an 82-kD protein with six homologous subdomains, referred to as S1-S6. Each subdomain is composed of a five-stranded β-sheet, flanked by two α-helices, one positioned perpendicular with respect to the strands and one positioned parallel. The β-sheets of the three N-terminal subdomains (S1-S3) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
α-actinin
Actinin is a microfilament protein. Alpha-actinin-1 is necessary for the attachment of actin myofilaments to the Z-lines in skeletal muscle cells, and to the dense bodies in smooth muscle cells. The functional protein is an anti-parallel dimer, which cross-links the thin filaments in adjacent sarcomeres, and therefore coordinates contractions between sarcomeres in the horizontal axis. The non-sarcomeric alpha-actinins, encoded by ''ACTN1'' and ''ACTN4'', are widely expressed. ''ACTN2'' expression is found in both cardiac and skeletal muscle, whereas ''ACTN3'' is limited to the latter. Both ends of the rod-shaped alpha-actinin dimer contain actin-binding domains. Mutations in ''ACTN4'' can cause the kidney disease focal segmental glomerulosclerosis (FSGS). See also * Actin * Muscle contraction Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle ten ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropomyosin
Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in actin-based cytoskeletons. Tropomyosin and the actin skeleton All organisms contain organelles that provide physical integrity to their cells. These type of organelles are collectively known as the cytoskeleton, and one of the most ancient systems is based on filamentous polymers of the protein actin. A polymer of a second protein, tropomyosin, is an integral part of most actin filaments in animals. Tropomyosins are a large family of integral components of actin filaments that play a critical role in regulating the function of actin filaments in both muscle and nonmuscle cells. These proteins consist of rod-shaped coiled-coil hetero- or homo- dimers that lie along the α-helical groove of most actin filaments. Interaction occurs along the length of the actin filament, with dimers aligning in a head-to-tail fashion. Tropomyosins are often categorised into two groups, muscle tropomyosin isoforms and nonmus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin
Myosins () are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility. The first myosin (M2) to be discovered was in 1864 by Wilhelm Kühne. Kühne had extracted a viscous protein from skeletal muscle that he held responsible for keeping the tension state in muscle. He called this protein ''myosin''. The term has been extended to include a group of similar ATPases found in the cells of both striated muscle tissue and smooth muscle tissue. Following the discovery in 1973 of enzymes with myosin-like function in '' Acanthamoeba castellanii'', a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes. Although myosin was originally thought to be restricted to muscle cells (hence '' myo-''(s) + '' -in''), there is no single "myosin"; rather it is a very large superfamily of genes whose p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |