|
Cobaltous Chloride
Cobalt(II) chloride is an inorganic compound of cobalt and chlorine, with the formula . The compound forms several hydrates ·''n'', for ''n'' = 1, 2, 6, and 9. Claims of the formation of tri- and tetrahydrates have not been confirmed.M. T. Saugier, M. Noailly, R. Cohen-Adad, F. Paulik, and J. Paulik (1977): "Equilibres solide ⇄ liquide ⇆ vapeur du systeme binaire -" ''Journal of Thermal Analysis'', volume 11, issue 1, pages 87–100. Note: the lowest point of fig.6 is inconsistent with fig.7; probably should be at -27.8 C instead of 0 C. The anhydrous form is a blue crystalline solid; the dihydrate is purple and the hexahydrate is pink. Commercial samples are usually the hexahydrate, which is one of the most commonly used cobalt compounds in the lab. Properties Anhydrous At room temperature, anhydrous cobalt chloride has the cadmium chloride structure () (Rm) in which the cobalt(II) ions are octahedrally coordinated. At about 706 °C (20 degrees below the melting point) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be " miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Physics'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt(II) Carbonate
Cobalt(II) carbonate is the inorganic compound with the formula CoCO3. This reddish paramagnetic solid is an intermediate in the hydrometallurgy, hydrometallurgical purification of cobalt from its ores. It is an inorganic pigment, and a precursor to catalysts. Cobalt(II) carbonate also occurs as the rare red/pink mineral spherocobaltite. Preparation and structure It is prepared by combining solutions cobaltous sulfate and sodium bicarbonate: :CoSO4 + 2 NaHCO3 → CoCO3 + Na2SO4 + H2O + CO2 This reaction is used in the precipitation of cobalt from an extract of its roasted ores. CoCO3 adopts a structure like calcite, consisting of cobalt in an octahedral coordination geometry. Reactions Like most transition metal carbonates, cobalt carbonate is insoluble in water, but is readily attacked by mineral acids: :CoCO3 + 2 HCl + 5 H2O → [Co(H2O)6]Cl2 + CO2 It is used to prepare many coordination complexes. The reaction of cobalt(II) carbonate and acetylacetone in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt(II) Hydroxide
Cobalt(II) hydroxide or cobaltous hydroxide is the inorganic compound with the formula , consisting of divalent cobalt cations and hydroxide anions . The pure compound, often called the "beta form" (β-) is a pink solid insoluble in water. The name is also applied to a related compound, often called "alpha" or "blue" form (α-), which incorporates other anions in its molecular structure. This compound is blue and rather unstable.Xiaohe Liu, Ran Yi, Ning Zhang, Rongrong Shi, Xingguo Li, and Guanzhou Qiu (2008): "Cobalt hydroxide nanosheets and their thermal decomposition to cobalt oxide nanorings". ''Chemistry: An Asian Journal'', volume 3, issue 4, pages 732-738. Cobalt(II) hydroxide is most used as a drying agent for paints, varnishes, and inks, in the preparation of other cobalt compounds, as a catalyst and in the manufacture of battery electrodes. Preparation Cobalt(II) hydroxide precipitates as a solid when an alkali metal hydroxide is added to an aqueous solution of Co2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MX2(H2O)2
Interferon-induced GTP-binding protein Mx2 is a protein that in humans is encoded by the ''MX2'' gene. The protein encoded by this gene has a nuclear and a cytoplasmic form and is a member of both the dynamin family and the family of large GTPases. The nuclear form is localized in a granular pattern in the heterochromatin region beneath the nuclear envelope. A nuclear localization signal (NLS) is present at the amino terminal end of the nuclear form but is lacking in the cytoplasmic form due to use of an alternate translation start codon. Antiviral activity This protein is upregulated by interferon-alpha but does not contain the antiviral activity of a similar myxovirus resistance protein 1. MX2/MXB has antiviral activity against HIV-1 The subtypes of HIV include two major types, HIV type 1 (HIV-1) and HIV type 2 (HIV-2). HIV-1 is related to viruses found in chimpanzees and gorillas living in western Africa, while HIV-2 viruses are related to viruses found in the sooty ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aquo Ligand
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry . Their behavior underpins many aspects of environmental, biological, and industrial chemistry. This article focuses on complexes where water is the only ligand ("homoleptic aquo complexes"), but of course many complexes are known to consist of a mix of aquo and other ligands. Stoichiometry and structure Hexa-aquo complexes Most aquo complexes are mono-nuclear, with the general formula , with or 3; they have an octahedral structure. The water molecules function as Lewis bases, donating a pair of electrons to the metal ion and forming a dative covalent bond with it. Typical examples are listed in the following table. : Tutton's salts are crystalline compounds with the generic formula (where , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
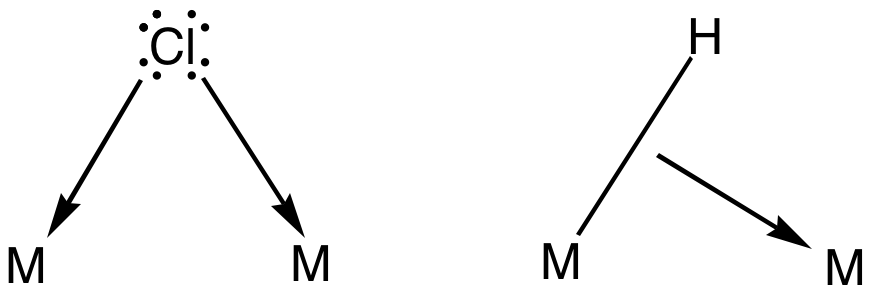
Bridging Chloride Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Polymer
A coordination polymer is an inorganic or organometallic polymer structure containing metal cation centers linked by ligands. More formally a coordination polymer is a coordination compound with repeating coordination entities extending in 1, 2, or 3 dimensions. It can also be described as a polymer whose repeat units are coordination complexes. Coordination polymers contain the subclass coordination networks that are coordination compounds extending, through repeating coordination entities, in 1 dimension, but with cross-links between two or more individual chains, loops, or spiro-links, or a coordination compound extending through repeating coordination entities in 2 or 3 dimensions. A subclass of these are the metal-organic frameworks, or MOFs, that are coordination networks with organic ligands containing potential voids. Coordination polymers are relevant to many fields, having many potential applications. Coordination polymers can be classified in a number of ways accor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deliquescent
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Of Crystallization
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation. Upon crystallization from water, or water-containing solvents, many compounds incorporate water molecules in their crystalline frameworks. Water of crystallization can generally be removed by heating a sample but the crystalline properties are often lost. Compared to inorganic salts, proteins crystallize with large amounts of water in the crystal lattice. A water content of 50% is not uncommon for proteins. Applications Knowledge of hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2.png)


_ion_in_aqueous_solution.jpg)