|
Co-crystal
Cocrystals are "solids that are crystalline single phase materials composed of two or more different molecular or ionic compounds generally in a stoichiometric ratio which are neither solvates nor simple salts." A broader definition is that cocrystals "consist of two or more components that form a unique crystalline structure having unique properties." Several subclassifications of cocrystals exist. Cocrystals can encompass many types of compounds, including hydrates, solvates and clathrates, which represent the basic principle of host–guest chemistry. Hundreds of examples of cocrystallization are reported annually. History The first reported cocrystal, quinhydrone, was studied by Friedrich Wöhler in 1844. Quinhydrone is a cocrystal of quinone and hydroquinone (known archaically as quinol). He found that this material was made up of a 1:1 molar combination of the components. Quinhydrone was analyzed by numerous groups over the next decade and several related cocrystals were made ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrates
In chemistry, a hydrate is a substance that contains water Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ... or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood. Chemical nature Inorganic chemistry Hydrates are inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal" that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain ''water of crystallization'' or ''water of hydration''. If the water is heavy water in which the constituent hydrogen is the isotope deuterium, then the term ''deuterate'' may be used in place of ''hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Diagrams
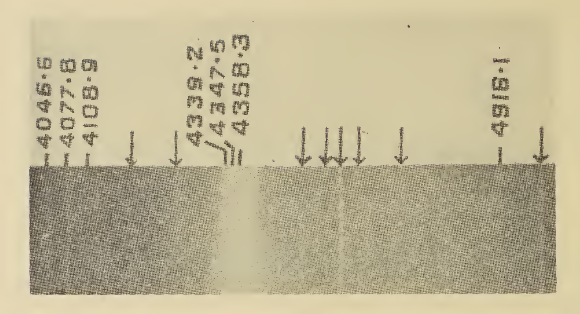
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium. Overview Common components of a phase diagram are ''lines of equilibrium'' or ''phase boundaries'', which refer to lines that mark conditions under which multiple phases can coexist at equilibrium. Phase transitions occur along lines of equilibrium. Metastable phases are not shown in phase diagrams as, despite their common occurrence, they are not equilibrium phases. Triple points are points on phase diagrams where lines of equilibrium intersect. Triple points mark conditions at which three different phases can coexist. For example, the water phase diagram has a triple point corresponding to the single temperature and pressure at which solid, liquid, and gaseous water can coexist in a stabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Racemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. History The first known racemic mixture was racemic acid, which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid. He manually separated the crystals of a mixture by hand, starting from an aqueous solution of the sodium ammonium salt of racemate tartaric acid. Pasteur benefited from the fact that ammonium tartrate salt that gives enantiomeric crystals with distinct crystal forms (at 77 °F). Reasoning from the macroscopic scale down to the molecular, he reckoned that the molecules had to have non-superimposable mirror images. A sample with only a single enantiomer is an ''enantiomerically pure'' or ''enantiopure'' compound. Etymology From racemic acid found in grapes; from Latin ''racemus'', meani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chiral (chemistry)
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek χείρ (''cheir'') 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physical properties, except that they often have opposite optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic, and it usually differs chemically and physically from the pure enantiomers. Chiral molecules will u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Raman Spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified. Raman spectroscopy relies upon inelastic scattering of photons, known as Raman scattering. A source of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range is used, although X-rays can also be used. The laser light interacts with molecular vibrations, phonons or other excitations in the system, resulting in the energy of the laser photons being shifted up or down. The shift in energy gives information about the vibrational modes in the system. Infrared spectroscopy typically yields similar yet complementary information. Typically, a sample is illuminated with a laser beam. Electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FT-IR
Fourier-transform infrared spectroscopy (FTIR) is a technique used to obtain an infrared Electromagnetic spectrum, spectrum of Absorption (electromagnetic radiation), absorption or Emission (electromagnetic radiation), emission of a solid, liquid, or gas. An FTIR spectrometer simultaneously collects high-resolution spectral data over a wide spectral range. This confers a significant advantage over a Dispersion (optics), dispersive spectrometer, which measures intensity over a narrow range of wavelengths at a time. The term ''Fourier-transform infrared spectroscopy'' originates from the fact that a Fourier transform (a mathematical process) is required to convert the raw data into the actual spectrum. Conceptual introduction The goal of absorption spectroscopy techniques (FTIR, Ultraviolet-visible spectroscopy, ultraviolet-visible ("UV-vis") spectroscopy, etc.) is to measure how much light a sample absorbs at each wavelength. The most straightforward way to do this, the "dispe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-Ray Diffraction
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercritical Fluid
A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist, but below the pressure required to compress it into a solid. It can effuse through porous solids like a gas, overcoming the mass transfer limitations that slow liquid transport through such materials. SCF are much superior to gases in their ability to dissolve materials like liquids or solids. Also, near the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be "fine-tuned". Supercritical fluids occur in the atmospheres of the gas giants Jupiter and Saturn, the terrestrial planet Venus, and probably in those of the ice giants Uranus and Neptune. Supercritical water is found on Earth, such as the water issuing from black smokers, a type of underwater hydrothermal vent. They are used as a substitute for organic solvents in a range of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ball Mill
A ball mill is a type of grinder used to grind or blend materials for use in mineral dressing processes, paints, pyrotechnics, ceramics, and selective laser sintering. It works on the principle of impact and attrition: size reduction is done by impact as the balls drop from near the top of the shell. A ball mill consists of a hollow cylindrical shell rotating about its axis. The axis of the shell may be either horizontal or at a small angle to the horizontal. It is partially filled with balls. The grinding media are the balls, which may be made of steel (chrome steel), stainless steel, ceramic, or rubber. The inner surface of the cylindrical shell is usually lined with an abrasion-resistant material such as manganese steel or rubber lining. Less wear takes place in rubber lined mills. The length of the mill is approximately equal to its diameter. The general idea behind the ball mill is an ancient one, but it was not until the industrial revolution and the invention of s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mortar And Pestle
Mortar and pestle is a set of two simple tools used from the Stone Age to the present day to prepare ingredients or substances by crushing and grinding them into a fine paste or powder in the kitchen, laboratory, and pharmacy. The ''mortar'' () is characteristically a bowl, typically made of hard wood, metal, ceramic, or hard stone such as granite. The ''pestle'' (, also ) is a blunt, club-shaped object. The substance to be ground, which may be wet or dry, is placed in the mortar where the pestle is pounded, pressed, and rotated into the substance until the desired texture is achieved. Mortars and pestles have been used in cooking since prehistory; today they are typically associated with the profession of pharmacy due to their historical use in preparing medicines. They are used in chemistry settings for pulverizing small amounts of chemicals; in arts and cosmetics for pulverizing pigments, binders, and other substances; in ceramics for making grog; in masonry and in other typ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sublimation (phase Transition)
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point in its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase. Sublimation has also been used as a generic term to describe a solid-to-gas transition (sublimation) followed by a gas-to-solid transition ( deposition). While vaporization from liquid to gas occurs as evaporation from the surface if it occurs below the boiling point of the liquid, and as boiling with formation of bubbles in the interior of the liquid if it occurs at the boiling point, there is no such distinction for the solid-to-gas transition which always occurs as sublimation from the surface. At ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seed Crystal
A seed crystal is a small piece of single crystal or polycrystal material from which a large crystal of typically the same material is grown in a laboratory. Used to replicate material, the use of seed crystal to promote growth avoids the otherwise slow randomness of natural crystal growth and allows manufacture on a scale suitable for industry. Crystal enlargement The large crystal can be grown by dipping the seed into a supersaturated solution, into molten material that is then cooled, or by growth on the seed face by passing vapor of the material to be grown over it. Theory The theory behind this effect is thought to derive from the physical intermolecular interaction that occurs between compounds in a supersaturated solution (or possibly vapor). In solution, liberated (soluble) molecules (solute) are free to move about in random flow. This random flow permits for the possibility of two or more molecular compounds to interact. This interaction can potentiate intermolecular fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_chloride.jpg)