|
Clausius
Rudolf Julius Emanuel Clausius (; 2 January 1822 – 24 August 1888) was a German physicist and mathematician and is considered one of the central founding fathers of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he gave the theory of heat a truer and sounder basis. His most important paper, "On the Moving Force of Heat", published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy. In 1870 he introduced the virial theorem, which applied to heat. Life Clausius was born in Köslin (now Koszalin, Poland) in the Province of Pomerania in Prussia. His father was a Protestant pastor and school inspector, and Rudolf studied in the school of his father. In 1838, he went to the Gymnasium in Stettin. Clausius graduated from the University of Berlin in 1844 where he had studied mathematics and physics since 1840 with, among others, Gustav Magnus, Peter Gustav Le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
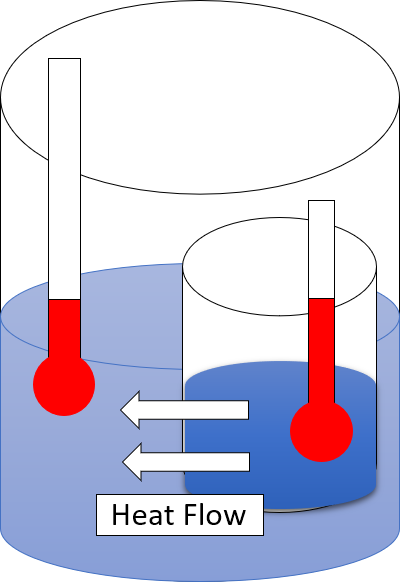
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unless energy in some form is supplied to reverse the direction of heat flow. Another definition is: "Not all heat energy can be converted into work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics in other versions establishes the concept of entropy as a physical property of a thermodynamic system. It can be used to predict whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. The second law may be formulated by the observation that the entropy of isolated systems left to spontaneous evolution cannot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clausius Theorem
The Clausius theorem (1855), also known as the ''Clausius inequality'', states that for a thermodynamic system (e.g. heat engine or heat pump) exchanging heat with external thermal reservoirs and undergoing a thermodynamic cycle, :-\oint dS_\text = \oint \frac \leq 0, where \oint dS_\text is the total entropy change in the external thermal reservoirs (surroundings), \delta Q is an infinitesimal amount of heat that is from each reservoir and absorbed by the system (\delta Q > 0 if heat from the reservoir is absorbed by the system, and \delta Q 0 if heat from the reservoir is absorbed by the system, and \delta Q 0) per thermodynamic cycle while in reversible cases, no entropy is created or added to the reservoirs. If the amount of energy added by heating can be measured during the process, and the temperature can be measured during the process, then the Clausius inequality can be used to determine whether the process is reversible or irreversible by carrying out the integration ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change, and information systems including the transmission of information in telecommunication. The thermodynamic concept was referred to by Scottish scientist and engineer William Rankine in 1850 with the names ''thermodynamic function'' and ''heat-potential''. In 1865, German physicist Rudolf Clausius, one of the leading founders of the field of thermodynamics, defined it as the quotient of an infinitesimal amount ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First Law Of Thermodynamics
The first law of thermodynamics is a formulation of the law of conservation of energy, adapted for thermodynamic processes. It distinguishes in principle two forms of energy transfer, heat and thermodynamic work for a system of a constant amount of matter. The law also defines the internal energy of a system, an extensive property for taking account of the balance of energies in the system. The law of conservation of energy states that the total energy of any isolated system, which cannot exchange energy or matter, is constant. Energy can be transformed from one form to another, but can be neither created nor destroyed. The first law for a thermodynamic process is often formulated asThe sign convention (Q is heat supplied ''to'' the system but W is work done ''by'' the system) is that of Rudolf Clausius (Equation IIa on page 384 of Clausius, R. (1850)), and it is followed below. :\Delta U = Q - W, where \Delta U denotes the change in the internal energy of a closed syste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disgregation
In the history of thermodynamics, disgregation is an early formulation of the concept of entropy. It was defined in 1862 by Rudolf Clausius as the magnitude of the degree in which the molecules of a body are separated from each other. Clausius modeled the concept on certain passages in French physicist Sadi Carnot's 1824 paper ''On the Motive Power of Fire'' which characterized the ''transformations'' of ''working substances'' (particles of a thermodynamic system) of an engine cycle, namely "mode of aggregation". The concept was later extended by Clausius in 1865 in the formulation of entropy, and in Ludwig Boltzmann's 1870s developments including the diversities of the motions of the microscopic constituents of matter, described in terms of order and disorder. In 1949, Edward Armand Guggenheim developed the concept of energy dispersal. The terms ''disgregation'' and ''dispersal'' are near in meaning. Historical context In 1824, French physicist Sadi Carnot assumed that heat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clausius–Clapeyron Relation
The Clausius–Clapeyron relation, named after Rudolf Clausius and Benoît Paul Émile Clapeyron, specifies the temperature dependence of pressure, most importantly vapor pressure, at a discontinuous phase transition between two phases of matter of a single constituent. Its relevance to meteorology and climatology is the increase of the water-holding capacity of the atmosphere by about 7% for every 1 °C (1.8 °F) rise in temperature. Definition On a pressure–temperature (''P''–''T'') diagram, the line separating the two phases is known as the coexistence curve. The Clapeyron relation gives the slope of the tangents to this curve. Mathematically, :\frac = \frac=\frac, where \mathrmP/\mathrmT is the slope of the tangent to the coexistence curve at any point, L is the specific latent heat, T is the temperature, \Delta v is the specific volume change of the phase transition, and \Delta s is the specific entropy change of the phase transition. The Clausius� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clausius–Duhem Inequality
The Clausius–Duhem inequality is a way of expressing the second law of thermodynamics that is used in continuum mechanics. This inequality is particularly useful in determining whether the constitutive relation of a material is thermodynamically allowable.. This inequality is a statement concerning the irreversibility of natural processes, especially when energy dissipation is involved. It was named after the German physicist Rudolf Clausius and French physicist Pierre Duhem. Clausius–Duhem inequality in terms of the specific entropy The Clausius–Duhem inequality can be expressed in integral form as : \frac\left(\int_\Omega \rho \eta \, dV\right) \ge \int_ \rho \eta \left(u_n - \mathbf\cdot\mathbf\right) dA - \int_ \frac~ dA + \int_\Omega \frac~dV. In this equation t is the time, \Omega represents a body and the integration is over the volume of the body, \partial \Omega represents the surface of the body, \rho is the mass density of the body, \eta is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clausius–Mossotti Relation
The Clausius–Mossotti relation expresses the dielectric constant (relative permittivity, ''ε''r) of a material in terms of the atomic polarizability, α, of the material's constituent atoms and/or molecules, or a homogeneous mixture thereof. It is named after Ottaviano-Fabrizio Mossotti and Rudolf Clausius. It is equivalent to the Lorentz–Lorenz equation. It may be expressed as: \frac = \frac where *\varepsilon_r = \varepsilon/\varepsilon_0 is the dielectric constant of the material, which for non-magnetic materials is equal to n^2 where n is the refractive index *\varepsilon_0 is the permittivity of free space *N is the number density of the molecules (number per cubic meter), and *\alpha is the molecular polarizability in SI-units (C·m2/V). In the case that the material consists of a mixture of two or more species, the right hand side of the above equation would consist of the sum of the molecular polarizability contribution from each species, indexed by ''i'' i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy Production
Entropy production (or generation) is the amount of entropy which is produced in any irreversible processes such as heat and mass transfer processes including motion of bodies, heat exchange, fluid flow, substances expanding or mixing, anelastic deformation of solids, and any irreversible thermodynamic cycle, including thermal machines such as power plants, heat engines, refrigerators, heat pumps, and air conditioners. In the dual representation entropy– exergy for accounting the second law of thermodynamics it can be expressed in equivalent terms of exergy disruption. Short history Entropy is produced in irreversible processes. The importance of avoiding irreversible processes (hence reducing the entropy production) was recognized as early as 1824 by Carnot. In 1865 Rudolf Clausius expanded his previous work from 1854 on the concept of "unkompensierte Verwandlungen" (uncompensated transformations), which, in our modern nomenclature, would be called the entropy produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theory Of Heat
The history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general. Owing to the relevance of thermodynamics in much of science and technology, its history is finely woven with the developments of classical mechanics, quantum mechanics, magnetism, and chemical kinetics, to more distant applied fields such as meteorology, information theory, and biology (physiology), and to technological developments such as the steam engine, internal combustion engine, cryogenics and electricity generation. The development of thermodynamics both drove and was driven by atomic theory. It also, albeit in a subtle manner, motivated new directions in probability and statistics; see, for example, the timeline of thermodynamics. History Contributions from antiquity The ancients viewed heat as that related to fire. In 3000 BC, the ancient Egyptians viewed heat as related to origin mythologies. The ancient Indian philos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |