|
Cinnamoyl
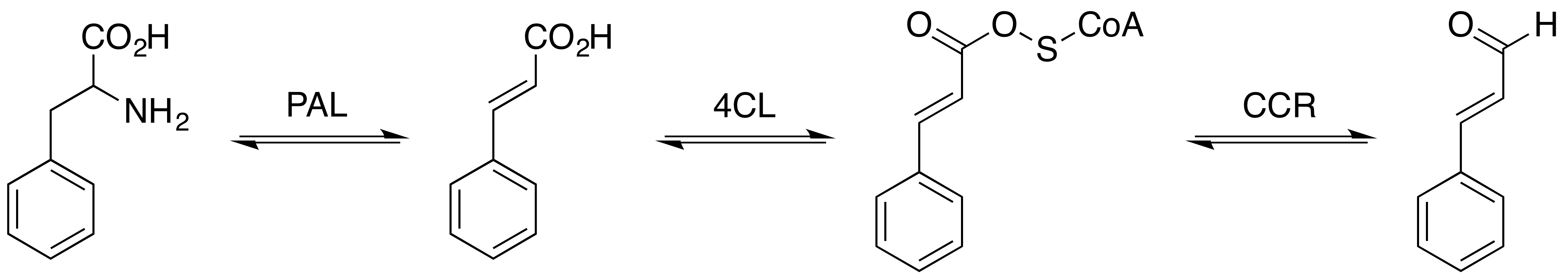
Cinnamic acid is an organic compound with the formula C6H5-CH=CH- COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a ''cis'' and a ''trans'' isomer, although the latter is more common. Occurrence and production Biosynthesis Cinnamic acid is a central intermediate in the biosynthesis of a myriad of natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. Its biosynthesis involves the action of the enzyme phenylalanine ammonia-lyase (PAL) on phenylalanine. Natural occurrence It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter. Cinnamic acid has a honey-like odor; it and its more volatile ethyl ester ( ethyl cinnamate) are flavor components ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamaldehyde
Cinnamaldehyde is an organic compound with the formula(C9H8O) C6H5CH=CHCHO. Occurring naturally as predominantly the ''trans'' (''E'') isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus ''Cinnamomum''. The essential oil of cinnamon bark is about 90% cinnamaldehyde. Cinnamaldehyde decomposes to styrene because of oxidation as a result of bad storage or transport conditions. Styrene especially forms in high humidity and high temperatures. This is the reason why cinnamon contains small amounts of styrene. Structure and synthesis Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is ''trans''-cinnamaldehyde. The molecule consists of a benzene rin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoic Acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source. Benzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates . History Benzoic acid was discovered in the sixteenth century. The dry distillation of gum benzoin was first described by Nostradamus (1556), and then by Alexius Pedemontanus (1560) and Blaise de Vigenère (1596). Justus von Liebig and Friedrich Wöhler determined the composition of benzoic acid. These latter also investigated how hippuric acid is related ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylpropanoid
The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. The coumaroyl component is produced from cinnamic acid. Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and also mediate plant-pollinator interactions as floral pigments and scent compounds. Hydroxycinnamic acids Phenylalanine is first converted to cinnamic aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. It is a colorless liquid with a characteristic almond-like odor. The primary component of bitter almond oil, benzaldehyde can be extracted from a number of other natural sources. Synthetic benzaldehyde is the flavoring agent in imitation almond extract, which is used to flavor cakes and other baked goods. History Benzaldehyde was first extracted in 1803 by the French pharmacist Martrès. His experiments focused on elucidating the nature of amygdalin, the poisonous material found in bitter almonds, the fruit of ''Prunus dulcis''. Further work on the oil by Pierre Robiquet and Antoine Boutron-Charlard, two French chemists, produced benzaldehyde. In 1832, Friedrich Wöhler and Justus von Liebig first synthesized benzaldehyde. Production As of 1999, 7000 tonnes of synthetic and 100 tonnes of natural b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl Chloride
Acetyl chloride (CH3COCl) is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl. Synthesis On an industrial scale, the reaction of acetic anhydride with hydrogen chloride produces a mixture of acetyl chloride and acetic acid: :(CH3CO)2O + HCl → CH3COCl + CH3CO2H Laboratory routes Acetyl chloride was first prepared in 1852 by French chemist Charles Gerhardt by treating potassium acetate with phosphoryl chloride. Acetyl chloride is produced in the laboratory by the reaction of acetic acid with chlorodehydrating agents such as PCl3, PCl5, SO2Cl2, phosgene, or SOCl2. However, these methods usually give acetyl chloride contaminated by phosphorus or sulfur impurities, which may interfere with the organic reactions. Other methods When heated, a mixture of dichloroacetyl chloride and acetic acid gives acetyl chloride. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Cinnamate
Ethyl cinnamate is the ester of cinnamic acid and ethanol. It is present in the essential oil of cinnamon. Pure ethyl cinnamate has a "fruity and balsamic odor, reminiscent of cinnamon with an amber note". The ''p''-methoxy derivative is reported to be a monoamine oxidase inhibitor. It can be synthesized by the esterification reaction involving ethanol and cinnamic acid in the presence of sulfuric acid. List of plants that contain the chemical * ''Kaempferia galanga ''Kaempferia galanga'', commonly known as kencur, aromatic ginger, sand ginger, cutcherry, is a monocotyledonous plant in the ginger family, and one of four plants called galangal. It is found primarily in open areas in Indonesia, southern Ch ...'' References Ethyl esters Phenyl compounds Food additives Flavors Phenylpropanoids {{ester-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Honey
Honey is a sweet and viscous substance made by several bees, the best-known of which are honey bees. Honey is made and stored to nourish bee colonies. Bees produce honey by gathering and then refining the sugary secretions of plants (primarily floral nectar) or the secretions of other insects, like the honeydew of aphids. This refinement takes place both within individual bees, through regurgitation and enzymatic activity, as well as during storage in the hive, through water evaporation that concentrates the honey's sugars until it is thick and viscous. Honey bees stockpile honey in the hive. Within the hive is a structure made from wax called honeycomb. The honeycomb is made up of hundreds or thousands of hexagonal cells, into which the bees regurgitate honey for storage. Other honey-producing species of bee store the substance in different structures, such as the pots made of wax and resin used by the stingless bee. Honey for human consumption is collected from wild ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shea Butter
Shea butter (, , or ; ) is a fat extracted from yellow the nut of the African shea tree (''Vitellaria paradoxa''). It is ivory in color when raw and commonly dyed yellow with borututu root or palm oil. It is widely used in cosmetics as a moisturizer, salve or lotion. Shea butter is edible and is used in food preparation in some African countries. Occasionally, shea butter is mixed with other oils as a substitute for cocoa butter, although the taste is noticeably different. The English word "shea" comes from , the tree's name in Bambara. It is known by many local names, such as in the Dagbani language, in the Wali language, in Twi, or in Hausa, in the Igbo language, in the Yoruba language, in the Wolof language of Senegal, and ''ori'' in some parts of West Africa and many others. History The common name is (lit. "shea tree") in the Bambara language of Mali. This is the origin of the English word, one pronunciation of which rhymes with "tea" , although the pronunci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Storax
Storax ( la, storax; el, στύραξ, ''stúrax''), often commercially sold as styrax, is a natural resin isolated from the wounded bark of ''Liquidambar orientalis'' Mill. (Asia Minor) and ''Liquidambar styraciflua'' L. (Central America) (Hamamelidaceae). It is distinct from benzoin (also called "storax"), a similar resin obtained from the Styracaceae plant family. Composition Purified storax contains circa 33 to 50% storesin, an alcoholic resin, both free and as cinnamic esters. Contains 5 to 15% cinnamic acid, 5 to 15% cinnamyl cinnamate, circa 10% phenylpropyl cinnamate; small amounts of ethyl cinnamate, benzyl cinnamate, and styrene, Some may contain traces of vanillin. Some sources report a resin containing triterpenic acids ( oleanolic and 3-epioleanolic acids). Uses Storax has a pleasant, floral/lilac, leathery, balsamic smell. Storax and its derivatives ( resinoid, essential oil, absolute) are used as flavors, fragrances, and in pharmaceuticals (Friar's Balsam). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Balsam
Balsam is the resinous exudate (or sap) which forms on certain kinds of trees and shrubs. Balsam (from Latin balsamum "gum of the balsam tree", ultimately from Semitic, Aramaic ''busma'', Arabic ''balsam'' and Hebrew ''basam'', "spice", "perfume") owes its name to the biblical Balm of Gilead. Chemistry Balsam is a solution of plant-specific resins in plant-specific solvents (essential oils). Such resins can include resin acids, esters, or alcohols. The exudate is a mobile to highly viscous liquid and often contains crystallized resin particles. Over time and as a result of other influences the exudate loses its liquidizing components or gets chemically converted into a solid material (i.e. by autoxidation). Some authors require balsams to contain benzoic or cinnamic acid or their esters. Plant resins are sometimes classified according to other plant constituents in the mixture, for example as: * pure resins (guaiac, hashish), * gum-resins (containing gums/polysaccharides) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamon
Cinnamon is a spice obtained from the inner bark of several tree species from the genus ''Cinnamomum''. Cinnamon is used mainly as an aromatic condiment and flavouring additive in a wide variety of cuisines, sweet and savoury dishes, breakfast cereals, snack foods, bagels, teas, and traditional foods. The aroma and flavour of cinnamon derive from its essential oil and principal component, cinnamaldehyde, as well as numerous other constituents including eugenol. Cinnamon is the name for several species of trees and the commercial spice products that some of them produce. All are members of the genus ''Cinnamomum'' in the family Lauraceae. Only a few ''Cinnamomum'' species are grown commercially for spice. ''Cinnamomum verum'' (AKA ''C. zeylanicum''), known as "Ceylon cinnamon" after its origins in Sri Lanka (formerly Ceylon), is considered to be "true cinnamon", but most cinnamon in international commerce is derived from four other species, usually and more correctly refe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the formula . It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino acid is classified as neutral, and nonpolar because of the inert and hydrophobic nature of the benzyl side chain. The L-isomer is used to biochemically form proteins coded for by DNA. Phenylalanine is a precursor for tyrosine, the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the skin pigment melanin. It is encoded by the codons UUU and UUC. Phenylalanine is found naturally in the milk of mammals. It is used in the manufacture of food and drink products and sold as a nutritional supplement for its analgesic and antidepressant effects. It is a direct precursor to the neuromodulator phenethylamine, a commonly used dietary supplement. As an essential amino acid, phenylalanine is n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |