|
Chemically Synthesized
Chemical synthesis (chemical combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable. A chemical synthesis involves one or more compounds (known as ''reagents'' or ''reactants'') that will experience a transformation under certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing (" work-up") or purification procedure to isolate the final product. The amount produced by chemical synthesis is known as the '' reaction yield''. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity that could be produced based on t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hermann Kolbe
Adolph Wilhelm Hermann Kolbe (27 September 1818 – 25 November 1884) was a German chemist and academic, and a major contributor to the birth of modern organic chemistry. He was a professor at Marburg and Leipzig. Kolbe was the first to apply the term synthesis in a chemical context, and contributed to the philosophical demise of vitalism through synthesis of the organic substance acetic acid from carbon disulfide, and also contributed to the development of structural theory. This was done via modifications to the idea of "radicals" and accurate prediction of the existence of secondary and tertiary alcohols, and to the emerging array of organic reactions through his Kolbe electrolysis of carboxylate salts, the Kolbe-Schmitt reaction in the preparation of aspirin and the Kolbe nitrile synthesis. After studies with Wöhler and Bunsen, Kolbe was involved with the early internationalization of chemistry through work in London (with Frankland). He was elected to the Royal Sw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retrosynthetic Analysis
Retrosynthetic analysis is a technique for solving problems in the planning of organic syntheses. This is achieved by transforming a target molecule into simpler precursor structures regardless of any potential reactivity/interaction with reagents. Each precursor material is examined using the same method. This procedure is repeated until simple or commercially available structures are reached. These simpler/commercially available compounds can be used to form a synthesis of the target molecule. Retrosynthetic analysis was used as early as 1917 in Robinson's Tropinone total synthesis. Important conceptual work on retrosynthetic analysis was published by George Vladutz in 1963. E.J. Corey formalized and popularized the concept from 1967 onwards in his article ''General methods for the construction of complex molecules'' and his book ''The Logic of Chemical Synthesis''. The power of retrosynthetic analysis becomes evident in the design of a synthesis. The goal of retrosynthetic a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
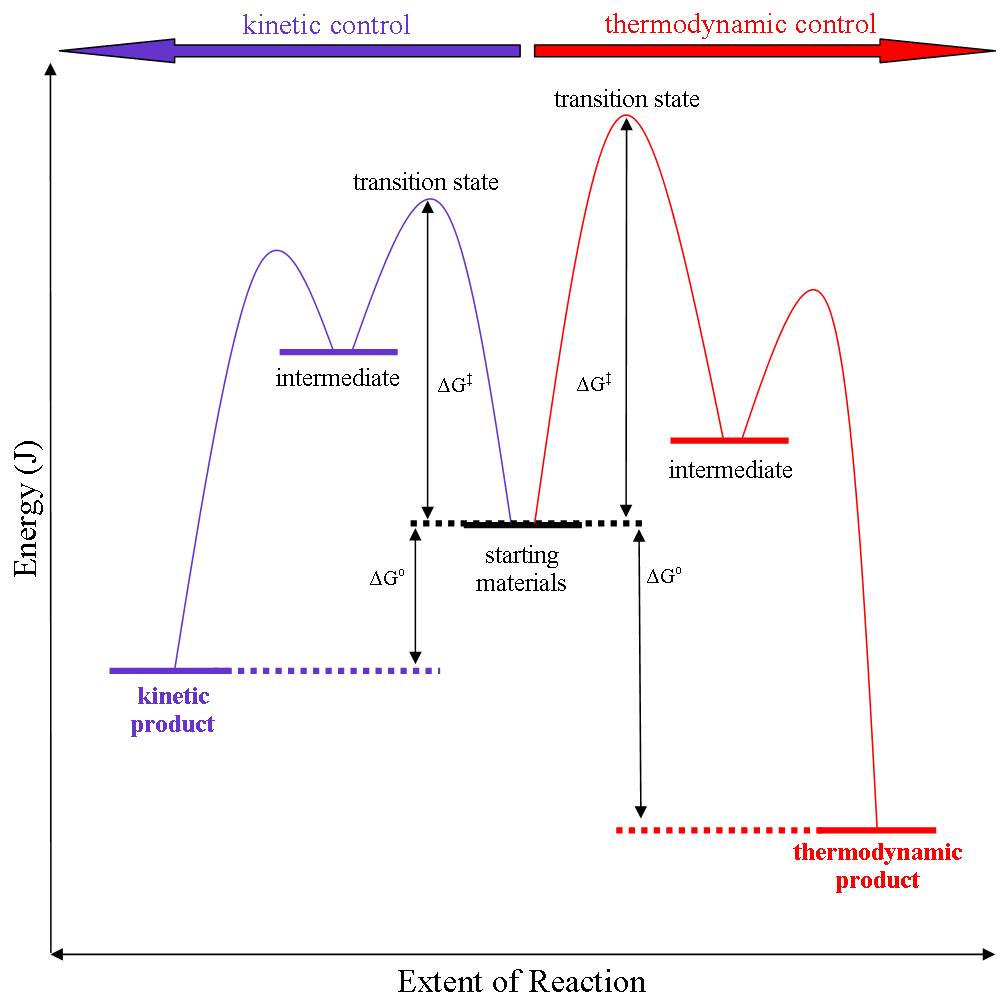
Thermodynamic Control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the conversion (chemistry), selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.Introduction to Organic Chemistry I, Seth Robert Elsheimer, Blackwell Publishing, 2000 The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protecting Group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis. In many preparations of delicate organic compounds, specific parts of the molecules cannot survive the required reagents or chemical environments. These parts (functional groups) must be protected. For example, lithium aluminium hydride is a highly reactive reagent that usefully reduces esters to alcohols. It always reacts with carbonyl groups, and cannot be discouraged by any means. When an ester must be reduced in the presence of a carbonyl, hydride attack on the carbonyl must be prevented. One way to do so converts the carbonyl into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the hydride step is complete, aqueous acid removes the acetal, restoring the carbonyl. This step ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemoselectivity
Chemoselectivity is the preferential reaction of a chemical reagent with one of two or more different functional groups. In a chemoselective system, a reagent in the presence of an aldehyde and an ester would mostly target the aldehyde, even if it has the option to react with the ester. Chemoselectivity is an area of interest in chemistry because scientists want to recreate complex biological compounds, such as natural products, and make specific modifications to them. Most chemical reactions bring together atoms that have negative charge character and atoms that have positive charge character. When evaluating possible reaction outcomes, several factors should be considered. The most important being identifying where in the molecule has the most electron density and where has the least. This analysis gives a good prediction of reactivity, but more factors such as connectivity, atomic orbital overlap, solvent effects, and the addition of supporting reagents can affect the reaction o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biocatalysis
Biocatalysis refers to the use of living (biological) systems or their parts to speed up ( catalyze) chemical reactions. In biocatalytic processes, natural catalysts, such as enzymes, perform chemical transformations on organic compounds. Both enzymes that have been more or less isolated and enzymes still residing inside living cells are employed for this task. Modern biotechnology, specifically directed evolution, has made the production of modified or non-natural enzymes possible. This has enabled the development of enzymes that can catalyze novel small molecule transformations that may be difficult or impossible using classical synthetic organic chemistry. Utilizing natural or modified enzymes to perform organic synthesis is termed chemoenzymatic synthesis; the reactions performed by the enzyme are classified as chemoenzymatic reactions. History Biocatalysis underpins some of the oldest chemical transformations known to humans, for brewing predates recorded history. The old ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoredox Catalysis
Photoredox catalysis is a branch of photochemistry that uses electron transfer, single-electron transfer. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes, and semiconductors. While organic photoredox catalysts were dominant throughout the 1990s and early 2000s, soluble transition-metal complexes are more commonly used today. Photochemistry of transition metal sensitizers Sensitizers absorb light to give redox-active excited states. For many metal-based sensitizers, excitation is realized as a metal-to-ligand charge transfer, whereby an electron moves from the metal (e.g., a d orbital) to an orbital localized on the ligands (e.g. the Pi bond, π* orbital of an aromatic ligand). This initial excited electronic state relaxes to a singlet excited state through Internal conversion (chemistry), internal conversion, a process where energy is dissipated as vibrational energy (heat) rather than as electromagnetic radiati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cascade Reaction
A cascade reaction, also known as a domino reaction or tandem reaction, is a chemical process that comprises at least two consecutive reactions such that each subsequent reaction occurs only in virtue of the chemical functionality formed in the previous step.Tietze, L. F.; Beifuss, U. ''Angew. Chem. Int. Ed.'' 1993, ''32'', 131–163. In cascade reactions, isolation of intermediates is not required, as each reaction composing the sequence occurs spontaneously. In the strictest definition of the term, the reaction conditions do not change among the consecutive steps of a cascade and no new reagents are added after the initial step.Padwa, A.; Bur, S. K. ''Tetrahedron'' 2007, ''63'', 5341–5378. By contrast, one-pot procedures similarly allow at least two reactions to be carried out consecutively without any isolation of intermediates, but do not preclude the addition of new reagents or the change of conditions after the first reaction. Thus, any cascade reaction is also a one-pot p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
One-pot Synthesis
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction in which a reactant is subjected to successive chemical reactions in just one reactor. This is much desired by chemists because avoiding a lengthy separation process and purification of the intermediate chemical compounds can save time and resources while increasing chemical yield. An example of a one-pot synthesis is the total synthesis of tropinone or the Gassman indole synthesis. Sequential one-pot syntheses can be used to generate even complex targets with multiple stereocentres, such as oseltamivir, which may significantly shorten the number of steps required overall and have important commercial implications. A sequential one-pot synthesis with reagents added to a reactor one at a time and without work-up is also called a telescoping synthesis. In one such procedure the reaction of 3-N-tosylaminophenol I with acrolein II affords a hydroxyl substituted quinoline III thro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Convergent Synthesis
In chemistry a convergent synthesis is a strategy that aims to improve the efficiency of multistep synthesis, most often in organic synthesis. In this type of synthesis several individual pieces of a complex molecule are synthesized in stage one, and then in stage two these pieces are combined to form the final product. In linear synthesis the overall yield quickly drops with each reaction step: :A → B → C → D Suppose the yield is 50% for each reaction; the overall yield of D is only 12.5% from A. In a convergent synthesis :A → B (50%) :C → D (50%) :B + D → E (25%) the overall yield of E (25%) looks much better. Convergent synthesis is applied in the synthesis of complex molecules and involves fragment coupling and independent synthesis. This technique is more useful if the compound is large and symmetric, where at least two aspects of the molecule can be formed separately and still come together. Examples: * Convergent synthesis is encountered in dendrimer syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |