Biocatalysis on:
[Wikipedia]
[Google]
[Amazon]
 Biocatalysis refers to the use of
Biocatalysis refers to the use of
 Biocatalyzed kinetic resolution is utilized extensively in the purification of racemic mixtures of synthetic amino acids. Many popular amino acid synthesis routes, such as the
Biocatalyzed kinetic resolution is utilized extensively in the purification of racemic mixtures of synthetic amino acids. Many popular amino acid synthesis routes, such as the  The maximum yield in such kinetic resolutions is 50%, since a yield of more than 50% means that some of wrong isomer also has reacted, giving a lower
The maximum yield in such kinetic resolutions is 50%, since a yield of more than 50% means that some of wrong isomer also has reacted, giving a lower  The
The 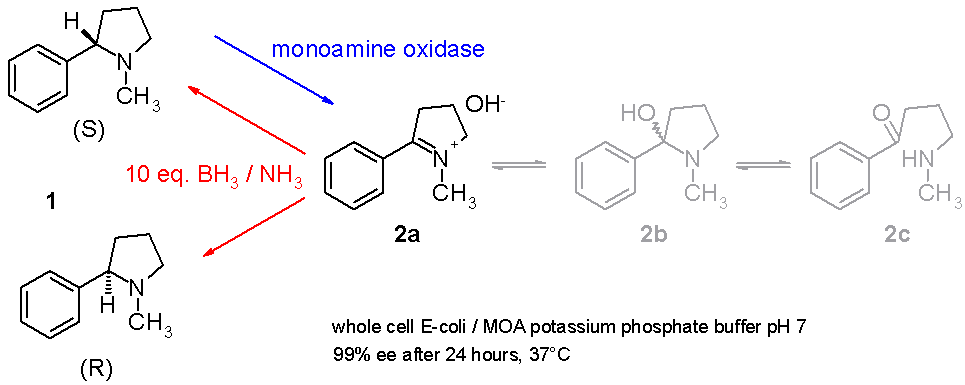
Nicotinamide adenine dinucleotide as a photocatalyst
. ''Science Advances''. 5 (7): eaax0501. do
10.1126/sciadv.aax0501
Austrian_Centre_of_Industrial_Biotechnology
_-_acib.html" ;"title="Austrian Centre of Industrial Biotechnology">Austrian Centre of Industrial Biotechnology
- acib">Austrian Centre of Industrial Biotechnology">Austrian Centre of Industrial Biotechnology
- acib
The Centre of Excellence for Biocatalysis - CoEBio3
The University of Exeter - Biocatalysis Centre
Center for Biocatalysis and Bioprocessing - The University of Iowa
TU Delft - Biocatalysis & Organic Chemistry (BOC)
KTH Stockholm - Biocatalysis Research Group
Institute of Technical Biocatalysis at the Hamburg University of Technology (TUHH)
Biocascades Project
{{Authority control Enzymes Organic chemistry Catalysis
 Biocatalysis refers to the use of
Biocatalysis refers to the use of living
Living or The Living may refer to:
Common meanings
*Life, a condition that distinguishes organisms from inorganic objects and dead organisms
** Living species, one that is not extinct
*Personal life, the course of an individual human's life
* ...
(biological) systems or their parts to speed up (catalyze
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
) chemical reactions. In biocatalytic processes, natural catalysts, such as enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s, perform chemical transformations on organic compounds
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The s ...
. Both enzymes that have been more or less isolated and enzymes still residing inside living cells are employed for this task. Modern biotechnology, specifically directed evolution
Directed evolution (DE) is a method used in protein engineering that mimics the process of natural selection to steer proteins or nucleic acids toward a user-defined goal. It consists of subjecting a gene to iterative rounds of mutagenesis ( ...
, has made the production of modified or non-natural enzymes possible. This has enabled the development of enzymes that can catalyze novel small molecule transformations that may be difficult or impossible using classical synthetic organic chemistry. Utilizing natural or modified enzymes to perform organic synthesis is termed chemoenzymatic synthesis; the reactions performed by the enzyme are classified as chemoenzymatic reactions.
History
Biocatalysis underpins some of the oldest chemical transformations known to humans, for brewing predates recorded history. The oldest records of brewing are about 6000 years old and refer to the Sumerians. The employment of enzymes and whole cells have been important for many industries for centuries. The most obvious uses have been in the food and drink businesses where the production of wine, beer, cheese etc. is dependent on the effects of the microorganisms. More than one hundred years ago, biocatalysis was employed to do chemical transformations on non-natural man-madeorganic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. T ...
s, with the last 30 years seeing a substantial increase in the application of biocatalysis to produce fine chemical
In chemistry, fine chemicals are complex, single, pure chemical substances, produced in limited quantities in multipurpose plants by multistep batch chemical or biotechnological processes. They are described by exacting specifications, used f ...
s, especially for the pharmaceutical industry
The pharmaceutical industry discovers, develops, produces, and markets drugs or pharmaceutical drugs for use as medications to be administered to patients (or self-administered), with the aim to cure them, vaccinate them, or alleviate symptoms. ...
.
Since biocatalysis deals with enzymes and microorganisms, it is historically classified separately from "homogeneous catalysis" and "heterogeneous catalysis". However, mechanistically speaking, biocatalysis is simply a special case of heterogeneous catalysis.
Advantages of chemoenzymatic synthesis
-Enzymes are environmentally benign, being completely degraded in the environment. -Most enzymes typically function under mild or biological conditions, which minimizes problems of undesired side-reactions such as decomposition,isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
, racemization In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic (optically inactive) form. This creates a 1:1 molar ratio of enantiomers and is referred too as a racemic mixture (i.e. conta ...
and rearrangement, which often plague traditional methodology.
-Enzymes selected for chemoenzymatic synthesis can be immobilized on a solid support. These immobilized enzymes demonstrate improved stability and re-usability.
-Through the development of protein engineering, specifically site-directed mutagenesis
Site-directed mutagenesis is a molecular biology method that is used to make specific and intentional mutating changes to the DNA sequence of a gene and any gene products. Also called site-specific mutagenesis or oligonucleotide-directed mutagenesi ...
and directed evolution, enzymes can be modified to enable non-natural reactivity. Modifications may also allow for a broader substrate range, enhance reaction rate or catalyst turnover.
-Enzymes exhibit extreme selectivity towards their substrates. Typically enzymes display three major types of selectivity:
*Chemoselectivity
Chemoselectivity is the preferential outcome of a chemical reaction over a set of possible alternative reactions.
In another definition, chemoselectivity refers to the selective reactivity of one functional group in the presence of others; often ...
: Since the purpose of an enzyme is to act on a single type of functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
, other sensitive functionalities, which would normally react to a certain extent under chemical catalysis, survive. As a result, biocatalytic reactions tend to be "cleaner" and laborious purification of product(s) from impurities emerging through side-reactions can largely be omitted.
*Regioselectivity
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base ...
and diastereoselectivity
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
: Due to their complex three-dimensional structure, enzymes may distinguish between functional groups which are chemically situated in different regions of the substrate molecule.
*Enantioselectivity
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
: Since almost all enzymes are made from L-amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
s, enzymes are chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
catalysts. As a consequence, any type of chirality present in the substrate molecule is "recognized" upon the formation of the enzyme-substrate complex. Thus a prochiral
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step. An achiral species which can be converted to a chiral in two steps is called proprochiral.
If two identical substituents are att ...
substrate may be transformed into an optically active product and both enantiomers of a racemic substrate may react at different rates.
These reasons, and especially the latter, are the major reasons why synthetic chemists have become interested in biocatalysis. This interest in turn is mainly due to the need to synthesize enantiopure
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
compounds as chiral building blocks for Pharmaceutical drug
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field an ...
s and agrochemicals.
Asymmetric biocatalysis
The use of biocatalysis to obtain enantiopure compounds can be divided into two different methods: # Kinetic resolution of a racemic mixture # Biocatalyzed asymmetric synthesis Inkinetic resolution
In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, resul ...
of a racemic mixture, the presence of a chiral object (the enzyme) converts one of the stereoisomers of the reactant into its product at a greater reaction rate than for the other reactant stereoisomer. The stereochemical mixture has now been transformed into a mixture of two different compounds, making them separable by normal methodology.
 Biocatalyzed kinetic resolution is utilized extensively in the purification of racemic mixtures of synthetic amino acids. Many popular amino acid synthesis routes, such as the
Biocatalyzed kinetic resolution is utilized extensively in the purification of racemic mixtures of synthetic amino acids. Many popular amino acid synthesis routes, such as the Strecker Synthesis
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with ammonia in the presence of potassium cyanide. The condensation reaction yields an α- ...
, result in a mixture of R and S enantiomers. This mixture can be purified by (I) acylating the amine using an anhydride and then (II) selectively deacylating only the L enantiomer using hog kidney acylase. These enzymes are typically extremely selective for one enantiomer leading to very large differences in rate, allowing for selective deacylation. Finally the two products are now separable by classical techniques, such as chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system ( ...
.
 The maximum yield in such kinetic resolutions is 50%, since a yield of more than 50% means that some of wrong isomer also has reacted, giving a lower
The maximum yield in such kinetic resolutions is 50%, since a yield of more than 50% means that some of wrong isomer also has reacted, giving a lower enantiomeric excess
In stereochemistry, enantiomeric excess (ee) is a measurement of purity used for chiral substances. It reflects the degree to which a sample contains one enantiomer in greater amounts than the other. A racemic mixture has an ee of 0%, while a si ...
. Such reactions must therefore be terminated before equilibrium is reached. If it is possible to perform such resolutions under conditions where the two substrate- enantiomers are racemizing continuously, all substrate may in theory be converted into enantiopure product. This is called dynamic resolution.
In biocatalyzed asymmetric synthesis, a non-chiral unit becomes chiral in such a way that the different possible stereoisomers are formed in different quantities. The chirality is introduced into the substrate by influence of enzyme, which is chiral. Yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constit ...
is a biocatalyst for the enantioselective reduction of ketones.
 The
The Baeyer–Villiger oxidation
The Baeyer–Villiger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant. The reaction is named after Adolf von Baeyer and Victor Villiger who ...
is another example of a biocatalytic reaction. In one study a specially designed mutant of ''Candida antarctica
''Candida antarctica'' is a yeast species in the genus '' Candida''.
''Candida antarctica'' is a source of important industrial enzymes. Immobilized ''Candida antarctica'' lipase can be used to catalyze the regioselective acylation of flavonoid ...
'' was found to be an effective catalyst for the Michael addition
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon ...
of acrolein with acetylacetone at 20 °C in absence of additional solvent.
Another study demonstrates how racemic nicotine
Nicotine is a natural product, naturally produced alkaloid in the nightshade family of plants (most predominantly in tobacco and ''Duboisia hopwoodii'') and is widely used recreational drug use, recreationally as a stimulant and anxiolytic. As ...
(mixture of S and R-enantiomers 1 in ''scheme 3'') can be deracemized in a one-pot
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction whereby a reactant is subjected to successive chemical reactions in just one reactor. This is much desired by chemists because avoiding a lengthy separ ...
procedure involving a monoamine oxidase isolated from Aspergillus niger
''Aspergillus niger'' is a mold classified within the ''Nigri'' section of the ''Aspergillus'' genus. The ''Aspergillus'' genus consists of common molds found throughout the environment within soil and water, on vegetation, in fecal matter, on de ...
which is able to oxidize only the amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
S-enantiomer to the imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
2 and involving an ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
–borane
Trihydridoboron, also known as borane or borine, is an unstable and highly reactive molecule with the chemical formula . The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated ...
reducing couple which can reduce the imine 2 back to the amine 1. In this way the S-enantiomer will continuously be consumed by the enzyme while the R-enantiomer accumulates. It is even possible to stereoinvert pure S to pure R.

Photoredox enabled biocatalysis
Recently, photoredox catalysis has been applied to biocatalysis, enabling unique, previously inaccessible transformations. Photoredox chemistry relies upon light to generatefree radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
intermediates. These radical intermediates are achiral thus racemic mixtures of product are obtained when no external chiral environment is provided. Enzymes can provide this chiral environment within the active site and stabilize a particular conformation and favoring formation of one, enantiopure product. Photoredox enabled biocatalysis reactions fall into two categories:
# Internal coenzyme/ cofactor photocatalyst
# External photocatalyst
Certain common hydrogen atom transfer (HAT
A hat is a head covering which is worn for various reasons, including protection against weather conditions, ceremonial reasons such as university graduation, religious reasons, safety, or as a fashion accessory. Hats which incorporate mecha ...
) cofactors ( NADPH and Flavin) can operate as single electron transfer (SET
Set, The Set, SET or SETS may refer to:
Science, technology, and mathematics Mathematics
*Set (mathematics), a collection of elements
*Category of sets, the category whose objects and morphisms are sets and total functions, respectively
Electro ...
) reagents. Although these species are capable of HAT without irradiation, their redox potentials are enhance by nearly 2.0 V upon visible light irradiation. When paired with their respective enzymes (typically ene-reductases) This phenomenon has been utilized by chemists to develop enantioselective reduction methodologies. For example medium sized lactams can be synthesized in the chiral environment of an ene-reductase through a reductive, baldwin favored, radical cyclization terminated by enatioselective HAT from NADPH.
The second category of photoredox enabled biocatalytic reactions use an external photocatalyst (PC). Many types of PCs with a large range of redox potentials can be utilized, allowing for greater tunability of reactive compared to using a cofactor. Rose bengal
Rose bengal (4,5,6,7-tetrachloro-2',4',5',7'-tetraiodofluorescein) is a stain. Rose bengal belongs to the class of organic compounds called xanthenes. Its sodium salt is commonly used in eye drops to stain damaged conjunctival and corneal cells ...
, and external PC, was utilized in tandem with an oxioreductase to enantioselectively deacylate medium sized alpha-acyl- ketones.
Using an external PC has some downsides. For example, external PCs typically complicate reaction design because the PC may react with both the bound and unbound substrate. If a reaction occurs between the unbound substrate and the PC, enantioselectivity is lost and other side reactions may occur.
Further reading
* * Kim, Jinhyun; Lee, Sahng Ha; Tieves, Florian; Paul, Caroline E.; Hollmann, Frank; Park, Chan Beum (5 July 2019).Nicotinamide adenine dinucleotide as a photocatalyst
. ''Science Advances''. 5 (7): eaax0501. do
10.1126/sciadv.aax0501
See also
* List of enzymes * Industrial enzymesReferences
External links
Austrian_Centre_of_Industrial_Biotechnology
_-_acib.html" ;"title="Austrian Centre of Industrial Biotechnology">Austrian Centre of Industrial Biotechnology
- acib">Austrian Centre of Industrial Biotechnology">Austrian Centre of Industrial Biotechnology
- acib
The Centre of Excellence for Biocatalysis - CoEBio3
The University of Exeter - Biocatalysis Centre
Center for Biocatalysis and Bioprocessing - The University of Iowa
TU Delft - Biocatalysis & Organic Chemistry (BOC)
KTH Stockholm - Biocatalysis Research Group
Institute of Technical Biocatalysis at the Hamburg University of Technology (TUHH)
Biocascades Project
{{Authority control Enzymes Organic chemistry Catalysis