|
Carothers Equation
In step-growth polymerization, the Carothers equation (or Carothers' equation) gives the degree of polymerization, , for a given fractional monomer conversion, . There are several versions of this equation, proposed by Wallace Carothers, who invented nylon in 1935. Linear polymers: two monomers in equimolar quantities The simplest case refers to the formation of a strictly linear polymer by the reaction (usually by condensation) of two monomers in equimolar quantities. An example is the synthesis of nylon-6,6 whose formula is from one mole of hexamethylenediamine, , and one mole of adipic acid, . For this case :\bar_n=\frac In this equation * is the number-average value of the degree of polymerization, equal to the average number of monomer units in a polymer molecule. For the example of nylon-6,6 \bar_n = 2n ( diamine units and diacid units). *p=\tfrac is the extent of reaction (or conversion to polymer), defined by ** is the number of molecules present initially as monome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Step-growth Polymerization
Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurring and some synthetic polymers are produced by step-growth polymerization, e.g. polyesters, polyamides, polyurethanes, etc. Due to the nature of the polymerization mechanism, a high extent of reaction is required to achieve high molecular weight. The easiest way to visualize the mechanism of a step-growth polymerization is a group of people reaching out to hold their hands to form a human chain—each person has two hands (= reactive sites). There also is the possibility to have more than two reactive sites on a monomer: In this case branched polymers production take place. IUPAC deprecates the term step-growth polymerization and recommends use of the terms polyaddition, when the propagation steps are addition reactions and no molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Harry R
Harry may refer to: TV shows *Harry (American TV series), ''Harry'' (American TV series), a 1987 American comedy series starring Alan Arkin *Harry (British TV series), ''Harry'' (British TV series), a 1993 BBC drama that ran for two seasons *Harry (talk show), ''Harry'' (talk show), a 2016 American daytime talk show hosted by Harry Connick Jr. People and fictional characters *Harry (given name), a list of people and fictional characters with the given name *Harry (surname), a list of people with the surname *Dirty Harry (musician) (born 1982), British rock singer who has also used the stage name Harry *Harry Potter (character), the main protagonist in a Harry Potter, Harry Potter fictional series by J. K. Rowling Other uses *Harry (derogatory term), derogatory term used in Norway *Harry (album), ''Harry'' (album), a 1969 album by Harry Nilsson *The tunnel used in the Stalag Luft III escape ("The Great Escape") of World War II *Harry (newspaper), ''Harry'' (newspaper), an undergrou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity quantifies the internal frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's axis than near its walls. Experiments show that some stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube with a constant rate of flow, the strength of the compensating force is proportional to the fluid's viscosity. In general, viscosity depends on a fluid's state, such as its temperature, pressure, and rate of deformation. However, the dependence on some of these properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Side Reaction
A side reaction is a chemical reaction that occurs at the same time as the actual main reaction, but to a lesser extent. It leads to the formation of by-product, so that the yield of main product is reduced: : + B ->[] P1 : + C ->[] P2 P1 is the main product if k1> k2. The by-product P2 is generally undesirable and must be Separation process, separated from the actual main product (usually in a Industrial separation processes, costly process). In organic synthesis B and C from the above equations usually represent different compounds. However, they could also just be different positions in the same molecule. A side reaction is also referred to as competing reaction when different compounds (B, C) compete for another reactant (A). If the side reaction occurs about as often as the main reaction, it is spoken of parallel reactions (especially in the kinetics, see below). Also there may be more complicated relationships: Compound A could reversibly but quickly react to substan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chain-growth Polymerization
Chain-growth polymerization ( AE) or chain-growth polymerisation ( BE) is a polymerization technique where unsaturated monomer molecules add onto the active site on a growing polymer chain one at a time. There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics. Introduction In 1953, Paul Flory first classified polymerization as "step-growth polymerization" and "chain-growth polymerization". IUPAC recommends to further simplify "chain-growth polymerization" to "chain polymerization". It is a kind of polymerization where an active center (free radical or ion) is formed, and a plurality of monomers can be polymerized together in a short period of time to form a macromolecule having a large molecular weight. In addition to the regenerated active sites of each monomer unit, polymer growth will only occur at one (or possibly more) endpoint. Many common polymers can be obtained by chain polymerization s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dispersity
In chemistry, the dispersity is a measure of the heterogeneity of sizes of molecules or particles in a mixture. A collection of objects is called uniform if the objects have the same size, shape, or mass. A sample of objects that have an inconsistent size, shape and mass distribution is called non-uniform. The objects can be in any form of chemical dispersion, such as particles in a colloid, droplets in a cloud, crystals in a rock, or polymer macromolecules in a solution or a solid polymer mass. Polymers can be described by molecular mass distribution; a population of particles can be described by size, surface area, and/or mass distribution; and thin films can be described by film thickness distribution. IUPAC has deprecated the use of the term ''polydispersity index'', having replaced it with the term ''dispersity'', represented by the symbol Đ (pronounced D-strokeStepto, R. F. T.; Gilbert, R. G.; Hess, M.; Jenkins, A. D.; Jones, R. G.; Kratochvíl P. (2009).Dispersity in P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weight Average Molecular Weight
The molar mass distribution (or molecular weight distribution) describes the relationship between the number of moles of each polymer species (Ni) and the molar mass (Mi) of that species. In linear polymers, the individual polymer chains rarely have exactly the same degree of polymerization and molar mass, and there is always a distribution around an average value. The molar mass distribution of a polymer may be modified by polymer fractionation. Definitions of molar mass average Different average values can be defined, depending on the statistical method applied. In practice, four averages are used, representing the weighted mean taken with the mole fraction, the weight fraction, and two other functions which can be related to measured quantities: *''Number average molar mass'' (Mn), also loosely referred to as ''number average molecular weight'' (NAMW). *''Mass average molar mass'' (Mw), where ''w'' stands for weight; also commonly referred to as ''weight average'' or ''w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Number Average Molecular Weight
The molar mass distribution (or molecular weight distribution) describes the relationship between the number of moles of each polymer species (Ni) and the molar mass (Mi) of that species. In linear polymers, the individual polymer chains rarely have exactly the same degree of polymerization and molar mass, and there is always a distribution around an average value. The molar mass distribution of a polymer may be modified by polymer fractionation. Definitions of molar mass average Different average values can be defined, depending on the statistical method applied. In practice, four averages are used, representing the weighted mean taken with the mole fraction, the weight fraction, and two other functions which can be related to measured quantities: *''Number average molar mass'' (Mn), also loosely referred to as ''number average molecular weight'' (NAMW). *''Mass average molar mass'' (Mw), where ''w'' stands for weight; also commonly referred to as ''weight average'' or ''wei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Branching (polymer Chemistry)
In polymer chemistry, branching is the regular or irregular attachment of side chains to a polymer's backbone chain. It occurs by the replacement of a substituent (e.g. a hydrogen atom) on a monomer subunit by another covalently-bonded chain of that polymer; or, in the case of a graft copolymer, by a chain of another type. Branched polymers have more compact and symmetrical molecular conformations, and exhibit intra-heterogeneous dynamical behavior with respect to the unbranched polymers. In crosslinking rubber by vulcanization, short sulfur branches link polyisoprene chains (or a synthetic variant) into a multiple-branched thermosetting elastomer. Rubber can also be so completely vulcanized that it becomes a rigid solid, so hard it can be used as the bit in a smoking pipe. Polycarbonate chains can be crosslinked to form the hardest, most impact-resistant thermosetting plastic, used in safety glasses. Branching may result from the formation of carbon-carbon or various ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limiting Reagent
The limiting reagent (or limiting reactant or limiting agent) in a chemical reaction is a reactant that is totally consumed when the chemical reaction is completed. The amount of product formed is limited by this reagent, since the reaction cannot continue without it. If one or more other reagents are present in excess of the quantities required to react with the limiting reagent, they are described as ''excess reagents'' or ''excess reactants'' (sometimes abbreviated as "xs"). The limiting reagent must be identified in order to calculate the percentage yield of a reaction since the theoretical yield is defined as the amount of product obtained when the limiting reagent reacts completely. Given the balanced chemical equation, which describes the reaction, there are several equivalent ways to identify the limiting reagent and evaluate the excess quantities of other reagents. Method 1: Comparison of reactant amounts This method is most useful when there are only two reactants. O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stoichiometry
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions. Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products, leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of the products can be empirically determined, then the amount of the other reactants can also be calculated. This is illustrated in the image here, where the balanced equation is: : Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. This particular chemical equation is an example of complete combust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
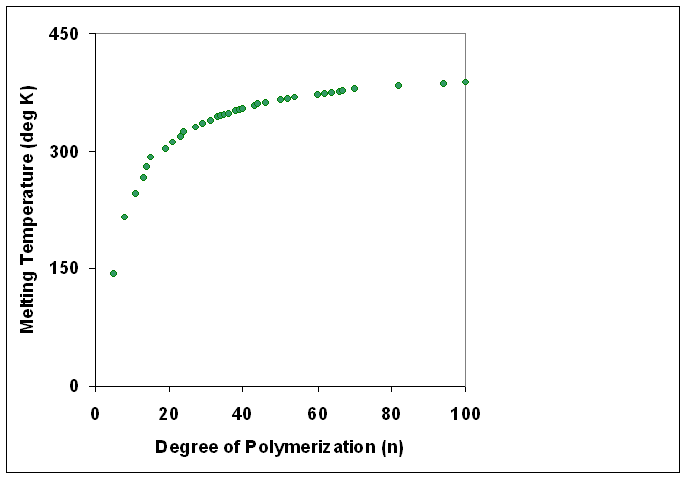
Degree Of Polymerization
The degree of polymerization, or DP, is the number of monomeric units in a macromolecule or polymer or oligomer molecule. For a homopolymer, there is only one type of monomeric unit and the ''number-average'' degree of polymerization is given by DP_n\equiv X_n=\frac, where Mn is the number-average molecular weight and M0 is the molecular weight of the monomer unit. For most industrial purposes, degrees of polymerization in the thousands or tens of thousands are desired. This number does not reflect the variation in molecule size of the polymer that typically occurs, it only represents the mean number of monomeric units. Some authors, however, define DP as the number of repeat units, where for copolymers the repeat unit may not be identical to the monomeric unit.Fried J.R. "Polymer Science and Technology" (Pearson Prentice-Hall, 2nd edn 2003), p.27 For example, in nylon-6,6, the repeat unit contains the two monomeric units —NH(CH2)6NH— and —OC(CH2)4CO—, so that a chain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |