|
Carbohydrate Conformation
Carbohydrate conformation refers to the overall three-dimensional structure adopted by a carbohydrate (saccharide) molecule as a result of the through-bond and through-space physical forces it experiences arising from its molecular structure. The physical forces that dictate the three-dimensional shapes of all molecules—here, of all monosaccharide, oligosaccharide, and polysaccharide molecules—are sometimes summarily captured by such terms as " steric interactions" and " stereoelectronic effects" (see below). Saccharide and other chemical conformations can be reasonably shown using two-dimensional structure representations that follow set conventions; these capture for a trained viewer an understanding of the three-dimensional structure via structure drawings (see organic chemistry article, and "3D Representations" section in molecular geometry article); they are also represented by stereograms on the two dimensional page, and increasingly using 3D display technologies on compu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or may not be different from ''n''), which does not mean the H has covalent bonds with O (for example with , H has a covalent bond with C but not with O). However, not all carbohydrates conform to this precise stoichiometric definition (e.g., uronic acids, deoxy-sugars such as fucose), nor are all chemicals that do conform to this definition automatically classified as carbohydrates (e.g. formaldehyde and acetic acid). The term is most common in biochemistry, where it is a synonym of saccharide (), a group that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Monosaccharides and disaccharides, the smallest (lower molecular wei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum ( spin) of a half-integer value, expressed in units of the reduced Planck constant, . Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Suffix
In linguistics, a suffix is an affix which is placed after the stem of a word. Common examples are case endings, which indicate the grammatical case of nouns, adjectives, and verb endings, which form the conjugation of verbs. Suffixes can carry grammatical information (inflectional suffixes) or lexical information ( derivational/lexical suffixes'').'' An inflectional suffix or a grammatical suffix. Such inflection changes the grammatical properties of a word within its syntactic category. For derivational suffixes, they can be divided into two categories: class-changing derivation and class-maintaining derivation. Particularly in the study of Semitic languages, suffixes are called affirmatives, as they can alter the form of the words. In Indo-European studies, a distinction is made between suffixes and endings (see Proto-Indo-European root). Suffixes can carry grammatical information or lexical information. A word-final segment that is somewhere between a free morpheme and a b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superscript
A subscript or superscript is a character (such as a number or letter) that is set slightly below or above the normal line of type, respectively. It is usually smaller than the rest of the text. Subscripts appear at or below the baseline, while superscripts are above. Subscripts and superscripts are perhaps most often used in formulas, mathematical expressions, and specifications of chemical compounds and isotopes, but have many other uses as well. In professional typography, subscript and superscript characters are not simply ordinary characters reduced in size; to keep them visually consistent with the rest of the font, typeface designers make them slightly heavier (i.e. medium or bold typography) than a reduced-size character would be. The vertical distance that sub- or superscripted text is moved from the original baseline varies by typeface and by use. In typesetting, such types are traditionally called "superior" and "inferior" letters, figures, etc., or just "superior ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prefix
A prefix is an affix which is placed before the Word stem, stem of a word. Adding it to the beginning of one word changes it into another word. For example, when the prefix ''un-'' is added to the word ''happy'', it creates the word ''unhappy''. Particularly in the study of languages, a prefix is also called a preformative, because it alters the form of the words to which it is affixed. Prefixes, like other affixes, can be either inflectional, creating a new form of the word with the same basic meaning and same part of speech, lexical category (but playing a different role in the sentence), or Morphological derivation, derivational, creating a new word with a new semantics, semantic meaning and sometimes also a different Part of speech, lexical category. Prefixes, like all other affixes, are usually Bound and unbound morphemes, bound morphemes. In English language, English, there are no inflectional prefixes; English uses suffixes instead for that purpose. The word ''prefix'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clockwise
Two-dimensional rotation can occur in two possible directions. Clockwise motion (abbreviated CW) proceeds in the same direction as a clock's hands: from the top to the right, then down and then to the left, and back up to the top. The opposite sense of rotation or revolution is (in Commonwealth English) anticlockwise (ACW) or (in North American English) counterclockwise (CCW). Terminology Before clocks were commonplace, the terms " sunwise" and "deasil", "deiseil" and even "deocil" from the Scottish Gaelic language and from the same root as the Latin "dexter" ("right") were used for clockwise. "Widdershins" or "withershins" (from Middle Low German "weddersinnes", "opposite course") was used for counterclockwise. The terms clockwise and counterclockwise can only be applied to a rotational motion once a side of the rotational plane is specified, from which the rotation is observed. For example, the daily rotation of the Earth is clockwise when viewed from above the South Pole, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an atom are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
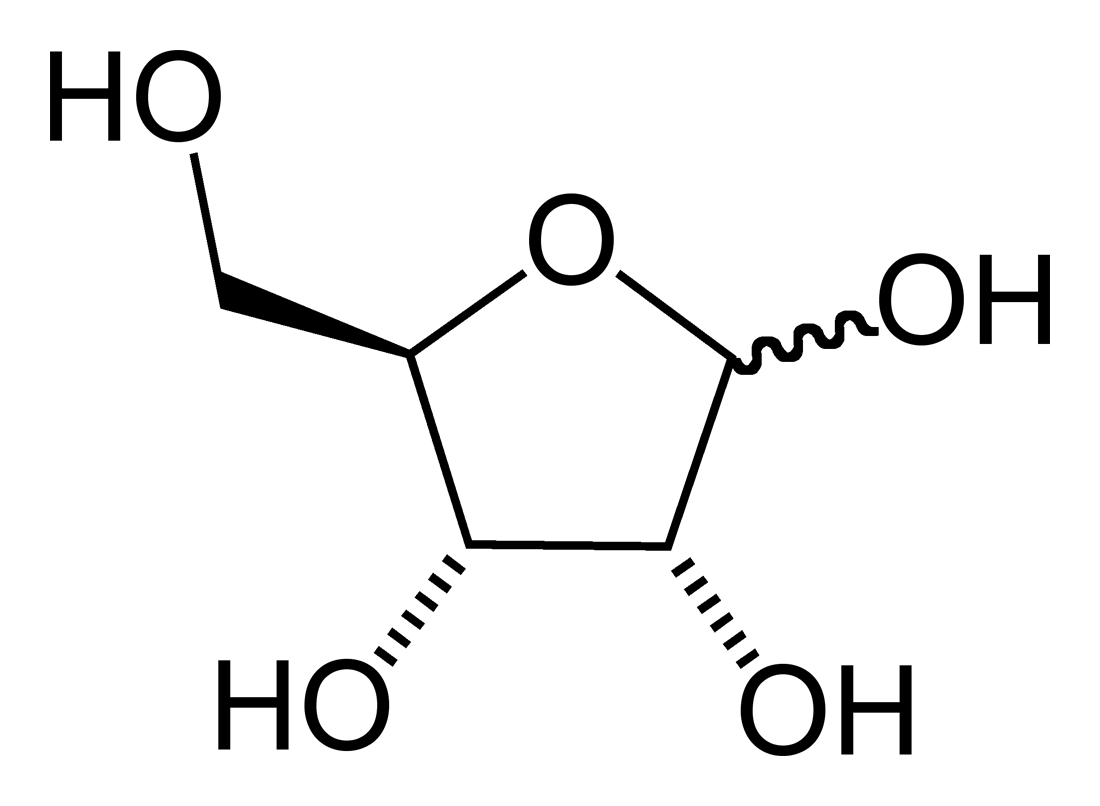
Furanose
A furanose is a collective term for carbohydrates that have a chemical structure that includes a five-membered ring system consisting of four carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle furan, but the furanose ring does not have double bonds. Structural properties The furanose ring is a cyclic hemiacetal of an aldopentose or a cyclic hemiketal of a ketohexose. A furanose ring structure consists of four carbon and one oxygen atom with the anomeric carbon to the right of the oxygen. The highest numbered chiral carbon (typically to the left of the oxygen in a Haworth projection) determines whether or not the structure has a -configuration or L-configuration. In an -configuration furanose, the substituent on the highest numbered chiral carbon is pointed downwards out of the plane, and in a D-configuration furanose, the highest numbered chiral carbon is facing upwards. The furanose ring will have either alpha or beta configuratio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyranose
Pyranose is a collective term for saccharides that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. There may be other carbons external to the ring. The name derives from its similarity to the oxygen heterocycle pyran, but the pyranose ring does not have double bonds. A pyranose in which the anomeric OH at C(l) has been converted into an OR group is called a pyranoside. Formation The pyranose ring is formed by the reaction of the hydroxyl group on carbon 5 (C-5) of a sugar with the aldehyde at carbon 1. This forms an intramolecular hemiacetal. If reaction is between the C-4 hydroxyl and the aldehyde, a furanose is formed instead. The pyranose form is thermodynamically more stable than the furanose form, which can be seen by the distribution of these two cyclic forms in solution. History Hermann Emil Fischer won the Nobel Prize in Chemistry (1902) for his work in determining the structure of the D-aldohexoses. How ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyranose Form
Pyranose is a collective term for saccharides that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. There may be other carbons external to the ring. The name derives from its similarity to the oxygen heterocycle pyran, but the pyranose ring does not have double bonds. A pyranose in which the anomeric OH at C(l) has been converted into an OR group is called a pyranoside. Formation The pyranose ring is formed by the reaction of the hydroxyl group on carbon 5 (C-5) of a sugar with the aldehyde at carbon 1. This forms an intramolecular hemiacetal. If reaction is between the C-4 hydroxyl and the aldehyde, a furanose is formed instead. The pyranose form is thermodynamically more stable than the furanose form, which can be seen by the distribution of these two cyclic forms in solution. History Hermann Emil Fischer won the Nobel Prize in Chemistry (1902) for his work in determining the structure of the D- aldohexoses. Ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furanose Forms
A furanose is a collective term for carbohydrates that have a chemical structure that includes a five-membered ring system consisting of four carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle furan, but the furanose ring does not have double bonds. Structural properties The furanose ring is a cyclic hemiacetal of an aldopentose or a cyclic hemiketal of a ketohexose. A furanose ring structure consists of four carbon and one oxygen atom with the anomeric carbon to the right of the oxygen. The highest numbered chiral carbon (typically to the left of the oxygen in a Haworth projection) determines whether or not the structure has a -configuration or L-configuration. In an -configuration furanose, the substituent on the highest numbered chiral carbon is pointed downwards out of the plane, and in a D-configuration furanose, the highest numbered chiral carbon is facing upwards. The furanose ring will have either alpha or beta configuratio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organisms
In biology, an organism () is any living system that functions as an individual entity. All organisms are composed of cells (cell theory). Organisms are classified by taxonomy into groups such as multicellular animals, plants, and fungi; or unicellular microorganisms such as protists, bacteria, and archaea. All types of organisms are capable of reproduction, growth and development, maintenance, and some degree of response to stimuli. Beetles, squids, tetrapods, mushrooms, and vascular plants are examples of multicellular organisms that differentiate specialized tissues and organs during development. A unicellular organism may be either a prokaryote or a eukaryote. Prokaryotes are represented by two separate domains – bacteria and archaea. Eukaryotic organisms are characterized by the presence of a membrane-bound cell nucleus and contain additional membrane-bound compartments called organelles (such as mitochondria in animals and plants and plastids in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |