|
Canyon Diablo (meteorite)
The Canyon Diablo meteorite refers to the many fragments of the asteroid that created Meteor Crater (also called Barringer Crater), Arizona, United States. Meteorites have been found around the crater rim, and are named for nearby Canyon Diablo, which lies about three to four miles west of the crater. History The impactor fell about 50,000 years ago. Initially known and used by pre-historic Native Americans, Canyon Diablo meteorites have been collected and studied by the scientific community since the 19th century. Meteor Crater, from the late 19th to the early 20th century, was the center of a long dispute over the origin of craters that showed little evidence of volcanism. That debate was largely settled by the early 1930s, thanks to work by Daniel M. Barringer, F.R. Moulton, Harvey Harlow Nininger, and Eugene Shoemaker. In 1953, Clair Cameron Patterson measured ratios of the lead isotopes in samples of the meteorite. Through U-Pb radiometric dating, a refined estima ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IAB Meteorites
IAB meteorites are a group of iron meteorites according to their overall composition and a group of primitive achondrites because of silicate inclusions that show a strong affinity to winonaites and chondrites. Description The IAB meteorites are composed of meteoric iron (kamacite and taenite) and silicate inclusions. Structurally they can be hexahedrites, fine to coarse octahedrites, or even ataxites. Most of them are octahedrite with medium to coarse taenite-lamella and distinct Widmanstätten patterning. The silicate inclusions are composed of low-Ca pyroxene, high-Ca pyroxene, olivine, plagioclase, troilite, graphite, different phosphates, meteoric iron and traces of daubréelite and chromite. This composition is very similar to the composition of winonaites, and it is therefore argued that the two groups share the same parent body. There are also similarities with the IIICD meteorites, but it is not yet clear whether they are also part of that parent body. Classification T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium–lead Dating
Uranium–lead dating, abbreviated U–Pb dating, is one of the oldest and most refined of the radiometric dating schemes. It can be used to date rocks that formed and crystallised from about 1 million years to over 4.5 billion years ago with routine precisions in the 0.1–1 percent range. The method is usually applied to zircon. This mineral incorporates uranium and thorium atoms into its crystal structure, but strongly rejects lead when forming. As a result, newly-formed zircon deposits will contain no lead, meaning that any lead found in the mineral is radiogenic. Since the exact rate at which uranium decays into lead is known, the current ratio of lead to uranium in a sample of the mineral can be used to reliably determine its age. The method relies on two separate decay chains, the uranium series from 238U to 206Pb, with a half-life of 4.47 billion years and the actinium series from 235U to 207Pb, with a half-life of 710 million years. Decay routes Uranium decays to lead via ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schreibersite
Schreibersite is generally a rare iron nickel phosphide mineral, , though common in iron-nickel meteorites. It has been found on Disko Island in Greenland and Illinois. Another name used for the mineral is rhabdite. It forms tetragonal crystals with perfect 001 cleavage. Its color ranges from bronze to brass yellow to silver white. It has a density of 7.5 and a hardness of 6.5 – 7. It is opaque with a metallic luster and a dark gray streak. It was named after the Austrian scientist Carl Franz Anton Ritter von Schreibers (1775–1852), who was one of the first to describe it from iron meteorites.Schreibersite Webmineral Schreibersite is reported from the Magura Meteorite, Arva-(present name – Orava), Slovak Republic; the |
Sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide. Chemical properties The sulfide ion, S2−, does not exist in aqueous alkaline solutions of Na2S. Instead sulfide converts to hydrosulfide: :S2− + H2O → SH− + OH− Upon treatment with an acid, sulfide salts convert to hydrogen sulfide: :S2− + H+ → SH− :SH− + H+ → H2S Oxidation of sulfide is a complicated process. Depending on the conditions, the oxidation can produce elemental sulfur, polysulfides, polythionates, sulfite, or sulfate. Metal sulfides react with halogens, forming sulfur and metal salts. :8 MgS + 8 I2 → S8 + 8 M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base Metal
A base metal is a common and inexpensive metal, as opposed to a precious metal such as gold or silver. In numismatics, coins often derived their value from the precious metal content; however, base metals have also been used in coins in the past and today. Specific definitions In contrast to noble metals, base metals may be distinguished by oxidizing or corroding relatively easily and reacting variably with diluted hydrochloric acid (HCl) to form hydrogen. Examples include iron, nickel, lead and zinc. Copper is also considered a base metal because it oxidizes relatively easily, although it does not react with HCl. In mining and economics, the term base metals refers to industrial non-ferrous metals excluding precious metals. These include copper, lead, nickel and zinc. The U.S. Customs and Border Protection agency is more inclusive in its definition of commercial base metals. Its list includes—in addition to copper, lead, nickel, and zinc—the following metals: iron and st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kamacite
Kamacite is an alloy of iron and nickel, which is found on Earth only in meteorites. According to the International Mineralogical Association (IMA) it is considered a proper nickel-rich variety of the mineral native iron. The proportion iron:nickel is between 90%:10% and 95%:5%; small quantities of other elements, such as cobalt or carbon may also be present. The mineral has a metallic luster, is gray and has no clear cleavage although its crystal structure is isometric-hexoctahedral. Its density is about 8 g/cm3 and its hardness is 4 on the Mohs scale. It is also sometimes called balkeneisen. The name was coined in 1861 and is derived from the Greek root ''καμακ-'' "kamak" or ''κάμαξ'' "kamaks", meaning vine-pole. It is a major constituent of iron meteorites (octahedrite and hexahedrite types). In the octahedrites it is found in bands interleaving with taenite forming Widmanstätten patterns. In hexahedrites, fine parallel lines called Neumann lines are often ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haxonite
Haxonite is an iron nickel carbide mineral found in iron meteorites and carbonaceous chondrites. It has a chemical formula of , crystallises in the cubic crystal system and has a Mohs hardness of - 6. It was first described in 1971, and named after Howard J. Axon (1924–1992), metallurgist at the University of Manchester, Manchester, England. Type locality (geology), Co-type localities are the Toluca meteorite, Xiquipilco, Mexico and the Canyon Diablo meteorite, Meteor Crater, Coconino County, Arizona, US. It occurs associated with kamacite, taenite, schreibersite, cohenite, pentlandite and magnetite. See also * Glossary of meteoritics References {{Meteorites Carbide minerals Iron minerals Nickel minerals Meteorite minerals Cubic minerals Native element minerals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular crystalline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lonsdaleite
Lonsdaleite (named in honour of Kathleen Lonsdale), also called hexagonal diamond in reference to the crystal structure, is an allotrope of carbon with a hexagonal lattice, as opposed to the cubical lattice of conventional diamond. It is found in nature in meteorite debris; when meteors containing graphite strike the Earth, the immense heat and stress of the impact transforms the graphite into diamond, but retains graphite's hexagonal crystal lattice. Lonsdaleite was first identified in 1967 from the Canyon Diablo meteorite, where it occurs as microscopic crystals associated with ordinary diamond. It is translucent and brownish-yellow and has an index of refraction of 2.40–2.41 and a specific gravity of 3.2–3.3 . Its hardness is theoretically superior to that of cubic diamond (up to 58% more), according to computational simulations, but natural specimens exhibited somewhat lower hardness through a large range of values (from 7–8 on Mohs hardness scale). The cause is sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
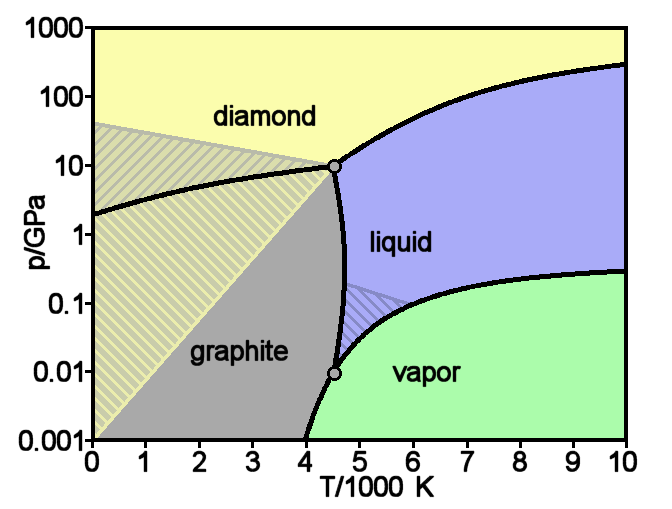
Diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the Chemical stability, chemically stable form of carbon at Standard conditions for temperature and pressure, room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest Scratch hardness, hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth. Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of lattice defect, defects or impurities (about one per million of lattice atoms) color diamond blue (bor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Daubréelite
Daubréelite is a rare sulfide mineral. It crystallizes with cubic symmetry and has chemical composition of Fe2+Cr3+2S4. It usually occurs as black platy aggregates. Naming and history Daubréelite was named after the French mineralogist, petrologist and meteoriticist Gabriel Auguste Daubrée. The mineral was first described in 1876 in the ''American Journal of Science''. Its type locality is the Coahuila meteorite, Bolsom de Mapimí, Coahuila, Mexico. Classification In the Nickel-Strunz classification daubréelite is part of the "Sulfides and Sulfosalts" and further a "metal sulfide with a metal-sulfide ratio of 3:4 and 2:3". Occurrences Daubréelite is found in iron meteorites as an inclusion in meteoric iron (kamacite and taenite). Further paragenetic minerals are alabandine, enstatite, graphite, plagioclase and schreibersite. According to one source daubréelite has been described from 34 localities. Some notable examples being the ALH 84001 meteorite, Hoba meteorite, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromite
Chromite is a crystalline mineral composed primarily of iron(II) oxide and chromium(III) oxide compounds. It can be represented by the chemical formula of FeCr2O4. It is an oxide mineral belonging to the spinel group. The element magnesium can substitute for iron in variable amounts as it forms a solid solution with magnesiochromite (MgCr2O4). A substitution of the element aluminium can also occur, leading to hercynite (FeAl2O4). Chromite today is mined particularly to make stainless steel through the production of ferrochrome (FeCr), which is an iron-chromium alloy. Chromite grains are commonly found in large mafic igneous intrusions such as the Bushveld in South Africa and India. Chromite is iron-black in color with a metallic luster, a dark brown streak and a hardness on the Mohs scale of 5.5. Properties Chromite minerals are mainly found in mafic-ultramafic igneous intrusions and are also sometimes found in metamorphic rocks. The chromite minerals occur in layered format ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |