|
Camphorsulfonic Acid
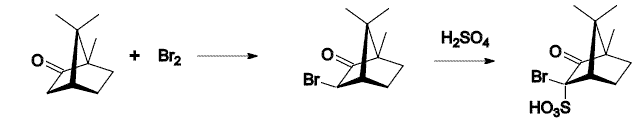
Camphorsulfonic acid, sometimes abbreviated CSA or 10-CSA is an organosulfur compound. Like typical sulfonic acids, it is a relatively strong acid that is a colorless solid at room temperature and is soluble in water and a wide variety of organic substances. This compound is commercially available. It can be prepared by sulfonation of camphor with sulfuric acid and acetic anhydride: : Although this reaction appears to be a sulfonation of an unactivated methyl group, the actual mechanism is believed to involve a retro- semipinacol rearrangement, deprotonation next to the tertiary carbocation to form an alkene, sulfonation of the alkene intermediate, and finally, semipinacol rearrangement to re-establish the ketone function. In organic synthesis, CSA and its derivatives can be used as resolving agents for chiral amines and other cations. The synthesis of osanetant was an example of this. 3-bromocamphor-8-sulfonic acid was used in the synthesis of enantiopure devazepide. Campho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur Compound
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two ( cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tellurium compounds. A classical chemical test for the detection of sulfur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonic Acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, , a tautomer of sulfurous acid, . Salts or esters of sulfonic acids are called sulfonates. Preparation Aryl sulfonic acids are produced by the process of sulfonation. Usually the sulfonating agent is sulfur trioxide. A large scale application of this method is the production of alkylbenzenesulfonic acids: :RC6H5 + SO3 -> RC6H4SO3H In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. Alkylsulfonic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Camphor
Camphor () is a waxy, colorless solid with a strong aroma. It is classified as a terpenoid and a cyclic ketone. It is found in the wood of the camphor laurel (''Cinnamomum camphora''), a large evergreen tree found in East Asia; and in the kapur tree ( ''Dryobalanops'' sp.), a tall timber tree from South East Asia. It also occurs in some other related trees in the laurel family, notably ''Ocotea usambarensis''. Rosemary leaves ('' Rosmarinus officinalis'') contain 0.05 to 0.5% camphor, while camphorweed (''Heterotheca'') contains some 5%. A major source of camphor in Asia is camphor basil (the parent of African blue basil). Camphor can also be synthetically produced from oil of turpentine. The compound is chiral, existing in two possible enantiomers as shown in the structural diagrams. The structure on the left is the naturally occurring (+)-camphor ((1''R'',4''R'')-bornan-2-one), while its mirror image shown on the right is the (−)-camphor ((1''S'',4''S'')-bornan-2-one) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air. Structure and properties Acetic anhydride, like most acid anhydrides, is a flexible molecule with a nonplanar structure. The pi system linkage through the central oxygen offers very weak resonance stabilization compared to the dipole-dipole repulsion between the two carbonyl oxygens. The energy barriers to bond rotation between each of the optimal aplanar conformations are quite low. Like most acid anhydrides, the carbonyl carbon atom of acetic anhydride has electrophilic character, as the leaving group is carboxylate. The internal asymmetry may contribute to acetic anhydride's potent electrophilicity as the asymmetric geome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
10-CSA Synthesis
1 (one, unit, unity) is a number representing a single or the only entity. 1 is also a numerical digit and represents a single unit of counting or measurement. For example, a line segment of ''unit length'' is a line segment of length 1. In conventions of sign where zero is considered neither positive nor negative, 1 is the first and smallest positive integer. It is also sometimes considered the first of the infinite sequence of natural numbers, followed by 2, although by other definitions 1 is the second natural number, following 0. The fundamental mathematical property of 1 is to be a multiplicative identity, meaning that any number multiplied by 1 equals the same number. Most if not all properties of 1 can be deduced from this. In advanced mathematics, a multiplicative identity is often denoted 1, even if it is not a number. 1 is by convention not considered a prime number; this was not universally accepted until the mid-20th century. Additionally, 1 is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semipinacol Rearrangement
The semipinacol rearrangement is a rearrangement reaction in organic chemistry involving a heterosubstituted alcohol of the type R1R2(HO)C–C(X)R3R4. The hetero substituent can be a halogen (Cl, Br, I), a tosylate, a mesylate or a thiol group. This reaction proceeds by removal of the leaving group X forming a carbocation as electron deficient center. One of the adjacent alkyl groups then migrates to the positive carbon in a 1,2-shift. Simultaneously with the shift, a pi bond forms from the oxygen to carbon, assisting in driving the migrating group off its position. The result is a ketone or aldehyde. In another definition all semipinacol rearrangements "''share a common reactive species in which an electrophilic carbon center, including but not limited to carbocations, is vicinal to an oxygen-containing carbon and can drive the 1,2-migration of a C–C or C–H bond to terminate the process, generating a carbonyl group'' ".''Semipinacol Rearrangement in Natural Product Synthesis' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: '' total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chiral Resolution
Chiral resolution, or enantiomeric resolution, is a process in stereochemistry for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active compounds, including drugs. Another term with the same meaning is optical resolution. The use of chiral resolution to obtain enantiomerically pure compounds has the disadvantage of necessarily discarding at least half of the starting racemic mixture. Asymmetric synthesis of one of the enantiomers is one means of avoiding this waste. Crystallization of diastereomeric salts The most common method for chiral resolution involves conversion of the racemic mixture to a pair of diastereomeric derivatives by reacting them with chiral derivatizing agents, also known as chiral resolving agents. The derivatives which are then separated by conventional crystallization, and converted back to the enantiomers by removal of the resolving agent. The process can be laborious and depends on the div ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osanetant
Osanetant (developmental code name SR-142,801) is a neurokinin 3 receptor antagonist which was developed by Sanofi-Synthélabo and was being researched for the treatment of schizophrenia but was discontinued. It was the first non-peptide NK3 antagonist developed in the mid-1990s. Professor David J. Anderson, Director and Leadership Chair of the Tianqiao and Chrissy Chen Institute for Neuroscience at California Institute of Technology, has advocated that osanetant be explored as a treatment for pain, anxiety, and aggression in humans and companion animals experiencing bereavement or social isolation, citing research suggesting that osanetant has an excellent safety profile and suppresses negative effects of social isolation in mice through an evolutionarily-conserved mechanism and without acting as a depressant. Another potential application for osanetant is in the treatment of drug addiction, as it has been found to block the effects of cocaine in animal models. Osanetant is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Devazepide
Devazepide (L-364,718, MK-329) is benzodiazepine drug, but with quite different actions from most benzodiazepines, lacking affinity for GABAA receptors and instead acting as an CCKA receptor antagonist. It increases appetite and accelerates gastric emptying, and has been suggested as a potential treatment for a variety of gastrointestinal problems including dyspepsia, gastroparesis and gastric reflux. It is also widely used in scientific research into the CCKA receptor. Synthesis Devazepide is synthesised in a similar manner to other benzodiazepines. See also *Benzodiazepine *Cholecystokinin antagonist A cholecystokinin receptor antagonist is a specific type of receptor antagonist which blocks the receptor sites for the peptide hormone cholecystokinin ( CCK). There are two subtypes of this receptor known at present, defined as CCKA and CCKB (a ... References {{Benzodiazepines Benzodiazepines Cholecystokinin antagonists Indoles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinolines
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance. Occurrence and isolation Quinoline was first extracted from coal tar in 1834 by German chemist Friedlieb Ferdinand Runge; he called quinoline ''leukol'' ("white oil" in Greek). Coal tar remains the principal source of commercial quinoline. In 1842, French chemist Charles Gerhardt obtained a compound by dry distilling quinine, st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |