|
COVI-VAC (U.S. COVID-19 Vaccine)
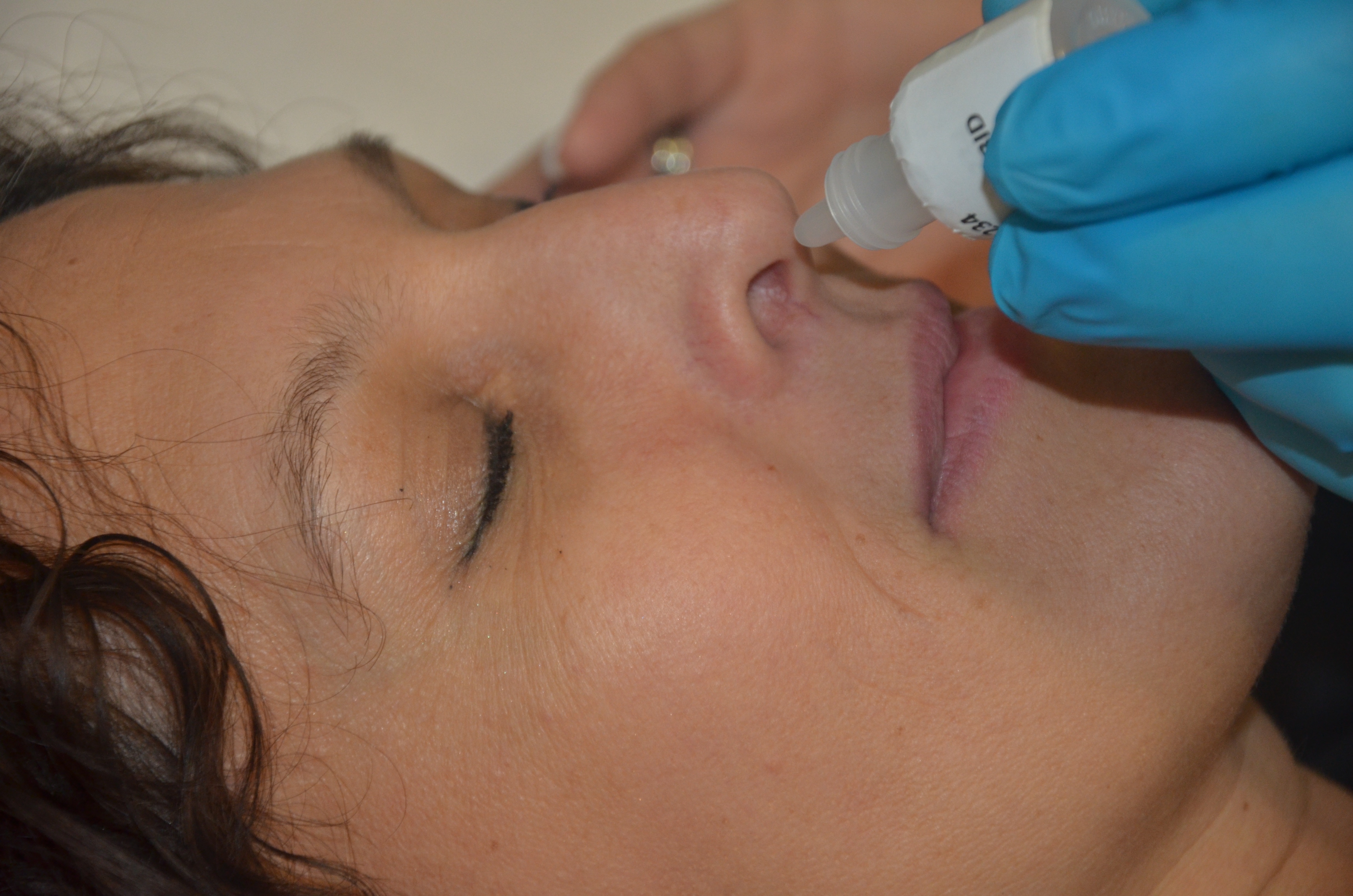
COVI-VAC ( codenamed CDX-005) is a COVID-19 vaccine A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the virus that causes coronavirus disease 2019 ( COVID19). Prior to the COVID19 pandemic, an e ... developed by Codagenix, Inc. In December 2020, COVI-VAC started a Phase I clinical trial, involving 48 participants. The trial was scheduled to complete in June 2021, with results to be reported by May 2022. On September 29, 2021, Codagenix presented positive phase 1 data for COVI-VAC at IDWEEK2021. Data indicates that COVI-VAC is well tolerated, with no significant adverse events reported and that administration of the intranasal vaccine was immunogenic and capable of blocking nasal replication of the virus with minimal viral shedding, recorded at levels lower than those likely to result in subsequent transmission of COVID-19. Furthermore, COVI-VAC was shown to stimulate both s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Severe Acute Respiratory Syndrome Coronavirus 2
Severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) is a strain of coronavirus that causes COVID-19 (coronavirus disease 2019), the respiratory illness responsible for the ongoing COVID-19 pandemic. The virus previously had a Novel coronavirus, provisional name, 2019 novel coronavirus (2019-nCoV), and has also been called the human coronavirus 2019 (HCoV-19 or hCoV-19). First identified in the city of Wuhan, Hubei, China, the World Health Organization declared the outbreak a public health emergency of international concern on January 30, 2020, and a pandemic on March 11, 2020. SARS‑CoV‑2 is a positive-sense single-stranded RNA virus that is Contagious disease, contagious in humans. SARS‑CoV‑2 is a virus of the species ''severe acute respiratory syndrome–related coronavirus'' (SARSr-CoV), related to the Severe acute respiratory syndrome coronavirus 1, SARS-CoV-1 virus that caused the 2002–2004 SARS outbreak. Despite its close relation to SARS-CoV-1, i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intranasal
Nasal administration, popularly known as snorting, is a route of administration in which drugs are insufflated through the nose. It can be a form of either topical administration or systemic administration, as the drugs thus locally delivered can go on to have either purely local or systemic effects. Nasal sprays are locally acting drugs such as decongestants for cold and allergy treatment, whose systemic effects are usually minimal. Examples of systemically active drugs available as nasal sprays are migraine drugs, rescue medications for overdose and seizure emergencies, nicotine replacement, and hormone treatments. Advantages with nasal systemic drug delivery The nasal cavity is covered by a thin mucosa which is well vascularised. Therefore, a drug molecule can be transferred quickly across the single epithelial cell layer directly to the systemic blood circulation without first-pass hepatic and intestinal metabolism. The effect is often reached within 5 min for smaller ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Codename
A code name, call sign or cryptonym is a Code word (figure of speech), code word or name used, sometimes clandestinely, to refer to another name, word, project, or person. Code names are often used for military purposes, or in espionage. They may also be used in industrial espionage, industrial counter-espionage to protect secret projects and the like from business rivals, or to give names to projects whose marketing name has not yet been determined. Another reason for the use of names and phrases in the military is that they transmit with a lower level of cumulative errors over a walkie-talkie or radio link than actual names. Military origins During First World War, World War I, names common to the Allies of World War I, Allies referring to nations, cities, geographical features, military units, military operations, diplomatic meetings, places, and individual persons were agreed upon, adapting pre-war naming procedures in use by the governments concerned. In the British case n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COVID-19 Vaccine
A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the virus that causes coronavirus disease 2019 ( COVID19). Prior to the COVID19 pandemic, an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). This knowledge accelerated the development of various vaccine platforms during early 2020. The initial focus of SARS-CoV-2 vaccines was on preventing symptomatic, often severe illness. In January 2020, the SARS-CoV-2 genetic sequence data was shared through GISAID, and by March 2020, the global pharmaceutical industry announced a major commitment to address COVID19. In 2020, the first COVID19 vaccines were developed and made available to the public through emergency authorizations and conditional approvals. Initially, most COVID19 vaccines were two ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase 1 Clinical Trial
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Summary Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population. Phase IV trials are 'post-marketing' or 'surveillance' studies conducted to monitor safety over severa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen. Each tip of the "Y" of an antibody contains a paratope (analogous to a lock) that is specific for one particular epitope (analogous to a key) on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can ''tag'' a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly (for example, by blocking a part of a virus that is essential for its invasion). To allow the immune system to recognize millions of different antigens, the antigen-binding sites at both tips of the antibody come in an equally wide variety. In contrast, the remainder of the antibody is relatively constant. It only occurs in a few varia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immune Response
An immune response is a reaction which occurs within an organism for the purpose of defending against foreign invaders. These invaders include a wide variety of different microorganisms including viruses, bacteria, parasites, and fungi which could cause serious problems to the health of the host organism if not cleared from the body. There are two distinct aspects of the immune response, the innate and the adaptive, which work together to protect against pathogens. The innate branch—the body's first reaction to an invader—is known to be a non-specific and quick response to any sort of pathogen. Components of the innate immune response include physical barriers like the skin and mucous membranes, immune cells such as neutrophils, macrophages, and monocytes, and soluble factors including cytokines and complement. On the other hand, the adaptive branch is the body's immune response which is catered against specific antigens and thus, it takes longer to activate the components involv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Attenuated Vaccine
An attenuated vaccine (or a live attenuated vaccine, LAV) is a vaccine created by reducing the virulence of a pathogen, but still keeping it viable (or "live"). Attenuation takes an infectious agent and alters it so that it becomes harmless or less virulent. These vaccines contrast to those produced by "killing" the virus (inactivated vaccine). Attenuated vaccines stimulate a strong and effective immune response that is long-lasting. In comparison to inactivated vaccines, attenuated vaccines produce a stronger and more durable immune response with a quick immunity onset. Attenuated vaccines function by encouraging the body to create antibodies and memory immune cells in response to the specific pathogen which the vaccine protects against. Common examples of live attenuated vaccines are measles, mumps, rubella, yellow fever, and some influenza vaccines. Development Attenuated viruses Viruses may be attenuated using the principles of evolution via serial passage of the virus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trials Related To COVID-19
Clinical may refer to: Healthcare * Of or about a clinic, a healthcare facility * Of or about the practice of medicine Other uses * ''Clinical'' (film), a 2017 American horror thriller See also * * * Clinical chemistry, the analysis of bodily fluids for diagnostic and therapeutic purposes * Clinical death, the cessation of blood circulation and breathing * Clinical formulation, a theoretically-based explanation of information obtained from clinical assessment * Clinical governance, a systematic approach to maintaining and improving the quality of patient care * Clinical linguistics, linguistics applied to speech-language pathology * Clinical psychology, the understanding, preventing, and relieving psychologically-based distress or dysfunction * Clinical research, to determine the safety and effectiveness of medications etc. * Clinical significance, the practical importance of a treatment effect * Clinical trial, experiments or observations done in clinical research * Clinical wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
American COVID-19 Vaccines
American(s) may refer to: * American, something of, from, or related to the United States of America, commonly known as the "United States" or "America" ** Americans, citizens and nationals of the United States of America ** American ancestry, people who self-identify their ancestry as "American" ** American English, the set of varieties of the English language native to the United States ** Native Americans in the United States, indigenous peoples of the United States * American, something of, from, or related to the Americas, also known as "America" ** Indigenous peoples of the Americas * American (word), for analysis and history of the meanings in various contexts Organizations * American Airlines, U.S.-based airline headquartered in Fort Worth, Texas * American Athletic Conference, an American college athletic conference * American Recordings (record label), a record label previously known as Def American * American University, in Washington, D.C. Sports teams Soccer * Ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)
