|
CDISC Migration
The Clinical Data Interchange Standards Consortium (CDISC) is a standards developing organization (SDO) dealing with medical research data linked with healthcare, to "enable information system interoperability to improve medical research and related areas of healthcare". The standards support medical research from protocol through analysis and reporting of results and have been shown to decrease resources needed by 60% overall and 70–90% in the start-up stages when they are implemented at the beginning of the research process. CDISC standards are harmonized through a model that is also a HL7 standard and is the process to becoming an ISO/ CEN standard. History * Late 1997 – Started as a Volunteer group * Summer 1998 – Invited to form DIA SIAC * 1999 – SDS v1.0; ODM v0.8 * 2000 – SDS v1.1 * Feb 2000 – Formed an Independent, non-profit organization * Dec 2001 – Global participation * 2001 – SDS v2.0; ODM v1.0 * 2002 – ODM v1.1; ADaM v1.0 * 2003 – LAB v1.0; SDTM ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard-developing Organization
A standards organization, standards body, standards developing organization (SDO), or standards setting organization (SSO) is an organization whose primary function is developing, coordinating, promulgating, revising, amending, reissuing, interpreting, or otherwise contributing to the usefulness of technical standards to those who employ them. Such an organization works to create uniformity across producers, consumers, government agencies, and other relevant parties regarding terminology, product specifications (e.g. size, including units of measure), protocols, and more. Its goals could include ensuring that Company A's external hard drive works on Company B's computer, an individual's blood pressure measures the same with Company C's sphygmomanometer as it does with Company D's, or that all shirts that should not be ironed have the same icon (a clothes iron crossed out with an X) on the label. Most standards are voluntary in the sense that they are offered for adoption by people ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronic Data Capture
An electronic data capture (EDC) system is a computerized system designed for the collection of clinical data in electronic format for use mainly in human clinical trials. EDC replaces the traditional paper-based data collection methodology to streamline data collection and expedite the time to market for drugs and medical devices. EDC solutions are widely adopted by pharmaceutical companies and contract research organizations (CRO). Typically, EDC systems provide: * a graphical user interface component for data entry * a validation component to check user data * a reporting tool for analysis of the collected data EDC systems are used by life sciences organizations, broadly defined as the pharmaceutical, medical device and biotechnology industries in all aspects of clinical research, but are particularly beneficial for late-phase (phase III-IV) studies and pharmacovigilance and post-market safety surveillance. EDC can increase data accuracy and decrease the time to collect data f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Research
Clinical research is a branch of healthcare science that determines the safety and effectiveness ( efficacy) of medications, devices, diagnostic products and treatment regimens intended for human use. These may be used for prevention, treatment, diagnosis or for relieving symptoms of a disease. Clinical research is different from clinical practice. In clinical practice established treatments are used, while in clinical research evidence is collected to establish a treatment. Overview The term "clinical research" refers to the entire bibliography of a drug/device/biologic, in fact any test article from its inception in the lab to its introduction to the consumer market and beyond. Once the promising candidate or the molecule is identified in the lab, it is subjected to pre-clinical studies or animal studies where different aspects of the test article (including its safety toxicity if applicable and efficacy, if possible at this early stage) are studied. In the United States ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SNOMED CT
SNOMED CT or SNOMED Clinical Terms is a systematically organized computer-processable collection of medical terms providing codes, terms, synonyms and definitions used in clinical documentation and reporting. SNOMED CT is considered to be the most comprehensive, multilingual clinical healthcare terminology in the world. The primary purpose of SNOMED CT is to encode the meanings that are used in health information and to support the effective clinical recording of data with the aim of improving patient care. SNOMED CT provides the core general terminology for electronic health records. SNOMED CT comprehensive coverage includes: clinical findings, symptoms, diagnoses, procedures, body structures, organisms and other etiologies, substances, pharmaceuticals, devices and specimens. SNOMED CT is maintained and distributed by SNOMED International, an international non-profit standards development organization, located in London, UK. SNOMED International is the trading name of the Intern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Systematized Nomenclature Of Medicine
The Systematized Nomenclature of Medicine (SNOMED) is a systematic, computer-processable collection of medical terms, in human and veterinary medicine, to provide codes, terms, synonyms and definitions which cover anatomy, diseases, findings, procedures, microorganisms, substances, etc. It allows a consistent way to index, store, retrieve, and aggregate medical data across specialties and sites of care. Although now international, SNOMED was started in the U.S. by the College of American Pathologists (CAP) in 1973 and revised into the 1990s. In 2002 CAP's SNOMED Reference Terminology (SNOMED RT) was merged with, and expanded by, the National Health Service's Clinical Terms Version 3 (previously known as the Read codes) to produce SNOMED CT. Versions of SNOMED released prior to 2001 were based on a multiaxial, hierarchical classification system. As in any such system, a disease may be located in a body organ (anatomy), which results in a code in a topography axis and may lead to m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LOINC
Logical Observation Identifiers Names and Codes (LOINC) is a database and universal standard for identifying medical laboratory observations. First developed in 1994, it was created and is maintained by the Regenstrief Institute, a US nonprofit medical research organization. LOINC was created in response to the demand for an electronic database for clinical care and management and is publicly available at no cost. It is endorsed by the American Clinical Laboratory Association. Since its inception, the database has expanded to include not just medical laboratory code names but also nursing diagnosis, nursing interventions, outcomes classification, and patient care data sets. Function ''LOINC'' applies universal code names and identifiers to medical terminology related to electronic health records. The purpose is to assist in the electronic exchange and gathering of clinical results (such as laboratory tests, clinical observations, outcomes management and research). LOINC has two m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Health Level 7
Health Level Seven or HL7 refers to a set of international standards for transfer of clinical and administrative data between software applications used by various healthcare providers. These standards focus on the application layer, which is "layer 7" in the OSI model. The HL7 standards are produced by Health Level Seven International, an international standards organization, and are adopted by other standards issuing bodies such as American National Standards Institute and International Organization for Standardization. Hospitals and other healthcare provider organizations typically have many different computer systems used for everything from billing records to patient tracking. All of these systems should communicate with each other (or "interface") when they receive new information, or when they wish to retrieve information, but not all do so. HL7 International specifies a number of flexible standards, guidelines, and methodologies by which various healthcare systems can c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Health Informatics Service Architecture
The European Committee for Standardization ( CEN) Standard Architecture for Healthcare Information Systems (ENV 12967), Health Informatics Service Architecture or HISA is a standard that provides guidance on the development of modular open information technology (IT) systems in the healthcare sector. Broadly, architecture standards outline frameworks which can be used in the development of consistent, coherent applications, databases and workstations. This is done through the definition of hardware and software construction requirements and outlining of protocols for communications. The HISA standard provides a formal standard for a service-oriented architecture (SOA), specific for the requirements of health services, based on the principles of Open Distributed Processing. The HISA standard evolved from previous work on healthcare information systems architecture commenced by Reseau d’Information et de Communication Hospitalier Europeen (RICHE) in 1989, and subsequently built upon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ECTD
The electronic common technical document (eCTD) is an interface and international specification for the pharmaceutical industry to agency transfer of regulatory information. The specification is based on the Common Technical Document (CTD) format and was developed by the International Council for Harmonisation (ICH) Multidisciplinary Group 2 Expert Working Group (ICH M2 EWG). History Version 2.0 of eCTD – an upgrade over the original CTD – was finalized on February 12, 2002, and version 3.0 was finalized on October 8 of the same year. , the most current version is 3.2.2, released on July 16, 2008. A Draft Implementation Guide for version 4.0 of eCTD was released in August 2012. However, work stalled on the project. An additional Draft Implementation Guide was released in February 2015 Draft specifications and guides were issued in April 2016 by the ICH and the FDA, followed by a May 13 ICH "teleconference to discuss the guidance and any questions and clarifications needed." U. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Digital Imaging And Communications In Medicine
Digital Imaging and Communications in Medicine (DICOM) is the standard for the communication and management of medical imaging information and related data. DICOM is most commonly used for storing and transmitting medical images enabling the integration of medical imaging devices such as scanners, servers, workstations, printers, network hardware, and picture archiving and communication systems (PACS) from multiple manufacturers. It has been widely adopted by hospitals and is making inroads into smaller applications such as dentists' and doctors' offices. DICOM files can be exchanged between two entities that are capable of receiving image and patient data in DICOM format. The different devices come with DICOM Conformance Statements which state which DICOM classes they support. The standard includes a file format definition and a network communications protocol that uses TCP/IP to communicate between systems. The National Electrical Manufacturers Association (NEMA) holds the cop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
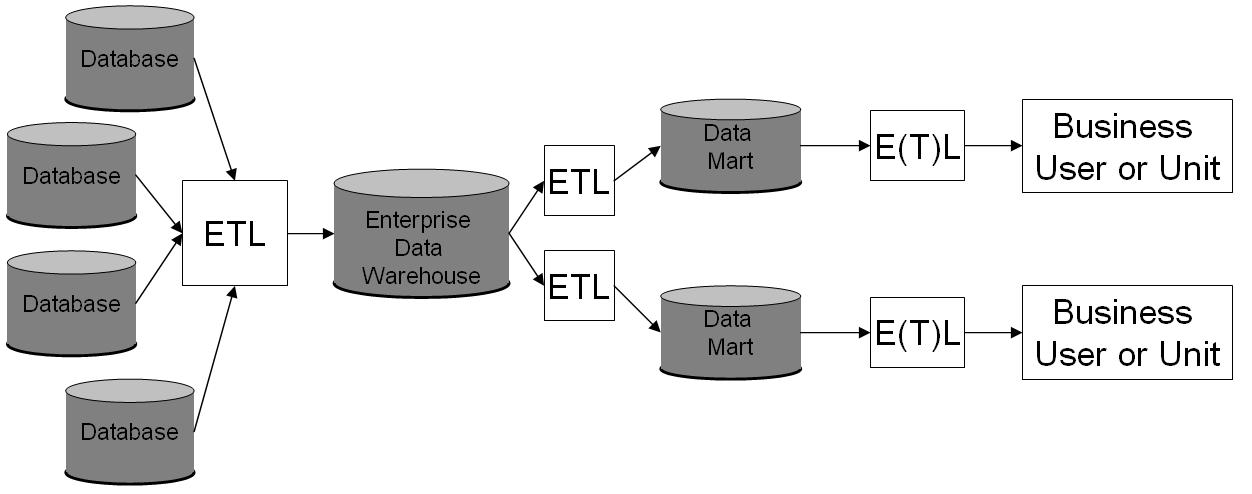
Data Warehouse
In computing, a data warehouse (DW or DWH), also known as an enterprise data warehouse (EDW), is a system used for Business reporting, reporting and data analysis and is considered a core component of business intelligence. DWs are central Repository (version control), repositories of integrated data from one or more disparate sources. They store current and historical data in one single place that are used for creating analytical reports for workers throughout the enterprise. The data stored in the warehouse is uploaded from the operational systems (such as marketing or sales). The data may pass through an operational data store and may require data cleansing for additional operations to ensure data quality before it is used in the DW for reporting. Extract, transform, load (ETL) and extract, load, transform (ELT) are the two main approaches used to build a data warehouse system. ETL-based data warehousing The typical extract, transform, load (ETL)-based data warehouse uses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Data Model
A data model is an abstract model that organizes elements of data and standardizes how they relate to one another and to the properties of real-world entities. For instance, a data model may specify that the data element representing a car be composed of a number of other elements which, in turn, represent the color and size of the car and define its owner. The term data model can refer to two distinct but closely related concepts. Sometimes it refers to an abstract formalization of the objects and relationships found in a particular application domain: for example the customers, products, and orders found in a manufacturing organization. At other times it refers to the set of concepts used in defining such formalizations: for example concepts such as entities, attributes, relations, or tables. So the "data model" of a banking application may be defined using the entity-relationship "data model". This article uses the term in both senses. A data model explicitly determines the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |