|
CD226
CD226 (Cluster of Differentiation 226), PTA1 (outdated term, 'platelet and T cell activation antigen 1') or DNAM-1 ( DNAX Accessory Molecule-1) is a ''~65 kDa'' immunoglobulin-like transmembrane glycoprotein expressed on the surface of natural killer cells, NK T cell, B cells, dendritic cells, hematopoietic precursor cells, platelets, monocytes and T cells. DNAM-1 gene ''CD226'' is conserved between human and mice. In humans the CD226 gene is located on chromosome 18q22.3. In mice the CD226 gene is located on chromosome 18E4. __NOTOC__ Structure DNAM-1 is composed of three domains: an extracellular domain of 230 amino acids with two immunoglobin-like V-set domains and eight N-glycosylation sites, a transmembrane domain of 28 amino acids and a cytosolic domain of 60 amino acids containing four putative tyrosine residues and one serine residue for phosphorylation. Signaling Upon engagement to its ligand, DNAM-1 is phosphorylated by protein kinase C. Then adhesive molecule ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DNAX
The τ and γ subunits are part of the DNA polymerase III holoenzyme of prokaryotes. The protein family is characterized by the well-conserved first N-terminal domain, approx. 365 amino acids. The eukaryotic equivalent to the DNA clamp loader is replication factor C, with the subunits RFC1 Replication factor C subunit 1 is a protein that in humans is encoded by the ''RFC1'' gene. Function The protein encoded by this gene is the large subunit of replication factor C, which is a five subunit DNA polymerase accessory protein. Replic ..., RFC2, RFC3, RFC4, and RFC5. The domain is also found in plants as gene STICHEL (STI), with similarity to cyanobacterial sequences. However, STI in plants is nuclear-localized and does not participate in genome duplication. It seems to instead regulate branching. References Bacterial proteins Protein families DNA replication {{molecular-biology-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Protein phosphorylation often activates (or deactivates) many enzymes. Glucose Phosphorylation of sugars is often the first stage in their catabolism. Phosphorylation allows cells to accumulate sugars because the phosphate group prevents the molecules from diffusing back across their transporter. Phosphorylation of glucose is a key reaction in sugar metabolism. The chemical equation for the conversion of D-glucose to D-glucose-6-phosphate in the first step of glycolysis is given by :D-glucose + ATP → D-glucose-6-phosphate + ADP :ΔG° = −16.7 kJ/mol (° indicates measurement at standard condition) Hepatic cells are freely permeable to glucose, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
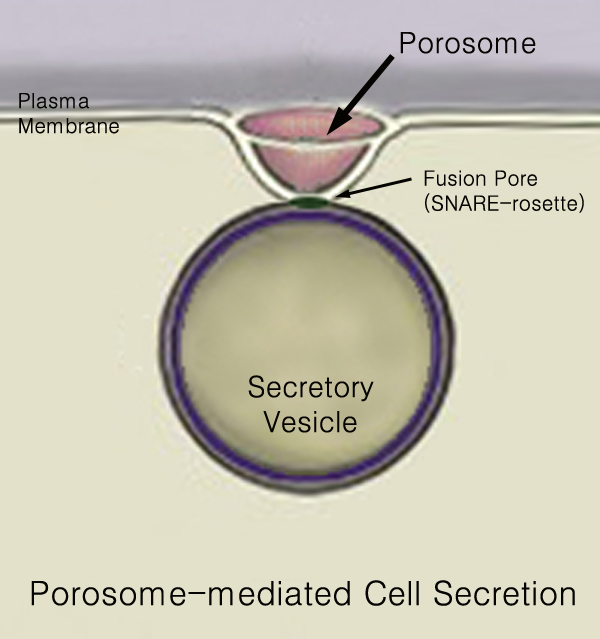
Secretion
440px Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mechanism of cell secretion is via secretory portals at the plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structures embedded in the cell membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell. Secretion in bacterial species means the transport or translocation of effector molecules for example: proteins, enzymes or toxins (such as cholera toxin in pathogenic bacteria e.g. '' Vibrio cholerae'') from across the interior (cytoplasm or cytosol) of a bacterial cell to its exterior. Secretion is a very important mechanism in bacterial functioning and operation in their natural surrounding environment for adaptation and survival. In eukaryotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Degranulation
Degranulation is a cellular process that releases antimicrobial cytotoxic or other molecules from secretory vesicles called granules found inside some cells. It is used by several different cells involved in the immune system, including granulocytes (neutrophils, basophils, and eosinophils) and mast cells. It is also used by certain lymphocytes such as natural killer (NK) cells and cytotoxic T cells, whose main purpose is to destroy invading microorganisms. Mast cells Antigens interact with IgE molecules already bound to high affinity Fc receptors on the surface of mast cells to induce degranulation, via the activation of tyrosine kinases within the cell. The mast cell releases a mixture of compounds, including histamine, proteoglycans, serotonin, and serine proteases from its cytoplasmic granules. Eosinophils In a similar mechanism, activated eosinophils release preformed mediators such as major basic protein, and enzymes such as peroxidase, following interaction between the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phospholipase
A phospholipase is an enzyme that hydrolyzes phospholipids into fatty acids and other lipophilic substances. Acids trigger the release of bound calcium from cellular stores and the consequent increase in free cytosolic Ca2+, an essential step in calcium signaling to regulate intracellular processes. There are four major classes, termed A, B, C, and D, which are distinguished by the type of reaction which they catalyze: *Phospholipase A ** Phospholipase A1 – cleaves the ''sn''-1 acyl chain (where ''sn'' refers to stereospecific numbering). **Phospholipase A2 – cleaves the ''sn''-2 acyl chain, releasing arachidonic acid. *Phospholipase B – cleaves both ''sn''-1 and ''sn''-2 acyl chains; this enzyme is also known as a lysophospholipase. *Phospholipase C – cleaves before the phosphate, releasing diacylglycerol and a phosphate-containing head group. PLCs play a central role in signal transduction, releasing the second messenger inositol triphosphate. * Phospholipase D – ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lymphocyte Cytosolic Protein 2
Lymphocyte cytosolic protein 2 (SH2 domain containing leukocyte protein of 76kDa), also known as LCP2 or SLP-76, is a signal-transducing adaptor protein expressed in T cells and myeloid cells and is important in the signaling of T-cell receptors (TCRs). As an adaptor protein, SLP-76 does not have catalytic functions, primarily binding other signaling proteins to form larger signaling complexes. It is a key component of the signaling pathways of receptors with immunoreceptor tyrosine-based activation motifs (ITAMs) such as T-cell receptors, its precursors, and receptors for the Fc regions of certain antibodies. SLP-76 is expressed in T-cells and related lymphocytes like natural killer cells. Structure and function The amino acid sequence of the protein has a central domain with a high concentration of prolines, as well as domains at the amino-terminal and carboxy-terminal of the amino acid sequence. The PDB file 1H3H depicts the SH3 domain of GRAP2 in complex with an RSTK- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SH2 Domain
The SH2 (Src Homology 2) domain is a structurally conserved protein domain contained within the Src oncoprotein and in many other intracellular signal-transducing proteins. SH2 domains allow proteins containing those domains to dock to phosphorylated tyrosine residues on other proteins. SH2 domains are commonly found in adaptor proteins that aid in the signal transduction of receptor tyrosine kinase pathways. Background SH2 is conserved by signalization of protein tyrosine kinase, which are binding on phosphotyrosine (pTyr). In the human proteome the class of pTyr-selective recognition domains is represented by SH2 domains. The N-terminal SH2 domains of cytoplasmic tyrosine kinase was at the beginning of evolution evolved with the occurrence of tyrosine phosphorylation. At the beginning it was supposed that, these domains serve as a substrate for their target kinase. Protein-protein interactions play a major role in cellular growth and development. Modular domains, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CD244
CD244 (Cluster of Differentiation 244) is a human protein encoded by the gene. It is also known as Natural Killer Cell Receptor 2B4 This gene encodes a cell surface receptor expressed on natural killer cells (NK cells) (and some T cells) mediating non-major histocompatibility complex (MHC) restricted killing. The interaction between NK-cell and target cells via this receptor is thought to modulate NK-cell cytolytic activity. Alternatively spliced transcript variants encoding different isoforms have been found for this gene. CD244 can also be expressed on non-lymphocytes such as eosinophils, mast cells and dendritic cells. See also * Cluster of differentiation The cluster of differentiation (also known as cluster of designation or classification determinant and often abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules providing targets for immunophen ... References Further reading * * * * * * * * * * * * * * * * * * * * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinase
In biochemistry, a kinase () is an enzyme that catalysis, catalyzes the transfer of phosphate groups from High-energy phosphate, high-energy, phosphate-donating molecules to specific Substrate (biochemistry), substrates. This process is known as phosphorylation, where the high-energy adenosine triphosphate, ATP molecule donates a phosphate group to the substrate (biology), substrate molecule. This transesterification produces a phosphorylated substrate and Adenosine diphosphate, ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and adenosine diphosphate, ADP gains a phosphate group (producing a dephosphorylated substrate and the high energy molecule of ATP). These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis. Kinases are part of the larger family of phosphotransferases. Kinases should not be confused with phosphorylases, which catalyze the addition of inorganic phosphate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components, microfilaments, intermediate filaments and microtubules, and these are all capable of rapid growth or disassembly dependent on the cell's requirements. A multitude of functions can be performed by the cytoskeleton. Its primary function is to give the cell its shape and mechanical resistance to deformation, and through association with extracellular connective tissue and other cells it stabilizes entire tissues. The cytoskeleton can also contract, thereby deforming the cell and the cell's environment and allowing cells to migrate. Moreover, it is involved in many cell signaling pathways and in the uptake of extracellular material (endocytosis), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actin
Actin is a protein family, family of Globular protein, globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in myofibril, muscle fibrils. It is found in essentially all Eukaryote, eukaryotic cells, where it may be present at a concentration of over 100 micromolar, μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm. An actin protein is the monomeric Protein subunit, subunit of two types of filaments in cells: microfilaments, one of the three major components of the cytoskeleton, and thin filaments, part of the Muscle contraction, contractile apparatus in muscle cells. It can be present as either a free monomer called G-actin (globular) or as part of a linear polymer microfilament called F-actin (filamentous), both of which are essential for such important cellular functions as the Motility, mobility and contraction of cell (biology), cells during cell division. Actin participates in many important cellular pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Raft
The cell membrane, plasma membranes of cells contain combinations of glycosphingolipids, cholesterol and protein Receptor (biochemistry), receptors organised in glycolipoprotein lipid microdomains termed lipid rafts. Their existence in cellular membranes remains somewhat controversial. It has been proposed that they are specialized membrane microdomains which compartmentalize cellular processes by serving as organising centers for the assembly of signaling molecules, allowing a closer interaction of protein receptors and their effectors to promote kinetically favorable interactions necessary for the signal transduction. Lipid rafts influence membrane fluidity and membrane protein Protein targeting, trafficking, thereby regulating neurotransmission and receptor trafficking. Lipid rafts are more ordered and tightly packed than the surrounding bilayer, but float freely within the membrane bilayer. Although more common in the cell membrane, lipid rafts have also been reported in other p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |