|
Borides
A boride is a compound between boron and a less electronegative element, for example silicon boride (SiB3 and SiB6). The borides are a very large group of compounds that are generally high melting and are covalent more than ionic in nature. Some borides exhibit very useful physical properties. The term boride is also loosely applied to compounds such as B12As2 (N.B. Arsenic has an electronegativity higher than boron) that is often referred to as icosahedral boride. Ranges of compounds The borides can be classified loosely as boron rich or metal rich, for example the compound YB66 at one extreme through to Nd2Fe14B at the other. The generally accepted definition is that if the ratio of boron atoms to metal atoms is 4:1 or more, the compound is boron rich; if it is less, then it is metal rich. Boron rich borides (B:M 4:1 or more) The main group metals, lanthanides and actinides form a wide variety of boron-rich borides, with metal:boron ratios up to YB66. The properties of this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is a meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmium Diboride
Osmium borides are compounds of osmium and boron. Their most remarkable property is potentially high hardness. It is thought that a combination of high electron density of osmium with the strength of boron-osmium covalent bonds will make osmium borides superhard materials, however this has not been demonstrated yet. For example, OsB2 is hard (hardness comparable to that of sapphire), but not superhard. Synthesis Osmium borides are produced in vacuum or inert atmosphere to prevent formation of osmium tetroxide Osmium tetroxide (also osmium(VIII) oxide) is the chemical compound with the formula OsO4. The compound is noteworthy for its many uses, despite its toxicity and the rarity of osmium. It also has a number of unusual properties, one being that the ..., which is a hazardous compound. Synthesis occurs at high temperatures (~1000 °C) from a mixture of MgB2 and OsCl3. Structure Three osmium borides are known: OsB, Os2B3 and OsB2. The first two have hexagonal structur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare-earth elements or rare-earth metals. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. There is some dispute on whether lanthanum or lutetium is a d-block element, but lutetium is usually considered so by those who study the matter; it is included due to its chemical similarities with the other 14. All lanthanide elements form trivalent cations, Ln3+, whose chemistry is largely determined by the ionic radius, which decreases steadily from lanthanum to lutetium. These elements are called lanthanides because the ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Diboride
Aluminium diboride (AlB2) is a chemical compound made from the metal aluminium and the metalloid boron. It is one of two compounds of aluminium and boron, the other being AlB12, which are both commonly referred to as aluminium boride. Structurally the B atoms form graphite-like sheets with Al atoms between them, and this is very similar to the structure of magnesium diboride. Single crystals of AlB2 exhibit metallic conductivity along the axis parallel to the basal hexagonal plane. Aluminium boride is considered a hazardous substance as it reacts with acids and hydrogen gas to produce toxic gases. For example, it reacts with hydrochloric acid to release borane and aluminium chloride. The crystal structure of AlB2 is often used as a prototype structure to describe intermetallic compounds. There are a large number of structure types that fall within the AlB2 structural family. See also *Boride A boride is a compound between boron and a less electronegative element, for exam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Diboride
Titanium diboride (TiB2) is an extremely hard ceramic which has excellent heat conductivity, oxidation stability and wear resistance. TiB2 is also a reasonable electrical conductor,J. Schmidt et al. "Preparation of titanium diboride TiB2 by spark plasma sintering at slow heating rate" Sci. Technol. Adv. Mater. 8 (2007) 37free download/ref> so it can be used as a cathode material in aluminium smelting and can be shaped by electrical discharge machining. Physical properties TiB2 shares some properties with boron carbide and titanium carbide, but many of its properties are superior to those of B4C & TiC: Exceptional hardness at extreme temperature *2nd hardest material at 3000°C (# diamond) *3rd hardest material at 2800°C (# cBN) *4th hardest material at 2100°C (# B4C) *5th hardest material at 1000°C (# B6O) Advantages over other borides *Highest boride elastic modulus *Highest boride fracture toughness *Highest boride compressive strength *2nd highest boride melting point (3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhenium Diboride
Rhenium diboride (ReB2) is a synthetic superhard material. It was first synthesized in 1962 and re-emerged recently due to hopes of achieving high hardness comparable to that of diamond. The reported ultrahigh hardness has been questioned, although this is a matter of definition as in the initial test rhenium diboride was able to scratch diamond. The production method of this material does not involve high pressures as with other hard synthetic materials, such as cubic boron nitride, which makes production cheap. However, rhenium itself is an expensive metal. The compound is formed from a mixture of rhenium, noted for its resistance to high pressure, and boron, which forms short, strong covalent bonds with rhenium. Synthesis ReB2 can be synthesized by at least three different methods at standard atmospheric pressure: solid-state metathesis, melting in an electric arc, and direct heating of the elements. In the metathesis reaction, rhenium trichloride and magnesium dib ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Diboride
Magnesium diboride is the inorganic compound with the formula MgB2. It is a dark gray, water-insoluble solid. The compound has attracted attention because it becomes superconducting at 39 K (−234 °C). In terms of its composition, MgB2 differs strikingly from most low-temperature superconductors, which feature mainly transition metals. Its superconducting mechanism is primarily described by BCS theory. Superconductivity Magnesium diboride's superconducting properties were discovered in 2001. Its critical temperature (''T''c) of is the highest amongst conventional superconductors. Among conventional ( phonon-mediated) superconductors, it is unusual. Its electronic structure is such that there exist two types of electrons at the Fermi level with widely differing behaviours, one of them ( sigma-bonding) being much more strongly superconducting than the other ( pi-bonding). This is at odds with usual theories of phonon-mediated superconductivity which assume that al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Boride
Titanium diboride (TiB2) is an extremely hard ceramic which has excellent heat conductivity, oxidation stability and wear resistance. TiB2 is also a reasonable electrical conductor,J. Schmidt et al. "Preparation of titanium diboride TiB2 by spark plasma sintering at slow heating rate" Sci. Technol. Adv. Mater. 8 (2007) 37free download/ref> so it can be used as a cathode material in aluminium smelting and can be shaped by electrical discharge machining. Physical properties TiB2 shares some properties with boron carbide and titanium carbide, but many of its properties are superior to those of B4C & TiC: Exceptional hardness at extreme temperature *2nd hardest material at 3000°C (# diamond) *3rd hardest material at 2800°C (# cBN) *4th hardest material at 2100°C (# B4C) *5th hardest material at 1000°C (# B6O) Advantages over other borides *Highest boride elastic modulus *Highest boride fracture toughness *Highest boride compressive strength *2nd highest boride melting point (3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum Hexaboride
Lanthanum hexaboride ( La B6, also called lanthanum boride and LaB) is an inorganic chemical, a boride of lanthanum. It is a refractory ceramic material that has a melting point of 2210 °C, and is insoluble in water and hydrochloric acid. It is extremely hard, with a Mohs hardness of 9.5. It has a low work function and one of the highest electron emissivities known, and is stable in vacuum. Stoichiometric samples are colored intense purple-violet, while boron-rich ones (above LaB6.07) are blue. Ion bombardment changes its color from purple to emerald green. LaB6 is a superconductor with a relatively low transition temperature of 0.45 K. Uses The principal use of lanthanum hexaboride is in hot cathodes, either as a single crystal or as a coating deposited by physical vapor deposition. Hexaborides, such as lanthanum hexaboride (LaB6) and cerium hexaboride (CeB6), have low work functions, around 2.5 eV. They are also somewhat resistant to cathode poisoning. Ceriu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hafnium Diboride
Hafnium diboride belongs to the class of ultra-high-temperature ceramics, a type of ceramic composed of hafnium and boron. It has a melting temperature of about 3250 °C. It is an unusual ceramic, having relatively high thermal and electrical conductivities, properties it shares with isostructural titanium diboride and zirconium diboride. It is a grey, metallic looking material. Hafnium diboride has a hexagonal crystal structure, a molar mass of 200.11 grams per mole, and a density of ~10.5 g/cm3. Hafnium diboride is often combined with carbon, boron, silicon, silicon carbide, and/or nickel to improve the consolidation of the hafnium diboride powder (sintering). It is commonly formed into a solid by a process called hot pressing, where the powders are pressed together using both heat and pressure. The material has potential for use in hypervelocity reentry vehicles such as ICBM heat shields or aerodynamic leading-edges, due to its strength and thermal properties. Unlike ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Boride
Silicon borides (also known as boron silicides) are lightweight ceramic compounds formed between silicon and boron. Several stoichiometric silicon boride compounds, SiB''n'', have been reported: silicon triboride, SiB3, silicon tetraboride, SiB4, silicon hexaboride, SiB6, as well as SiB''n'' (''n'' = 14, 15, 40, etc.). The ''n'' = 3 and ''n'' = 6 phases were reported as being co-produced together as a mixture for the first time by Henri Moissan and Alfred Stock in 1900 by briefly heating silicon and boron in a clay vessel. The tetraboride was first reported as being synthesized directly from the elements in 1960 by three independent groups: Carl Cline and Donald Sands; Ervin Colton; and Cyrill Brosset and Bengt Magnusson. It has been proposed that the triboride is a silicon-rich version of the tetraboride. Hence, the stoichiometry of either compound could be expressed as SiB4 - ''x'' where ''x'' = 0 or 1. All the silicon borides are black, crystalline materials of similar density: 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
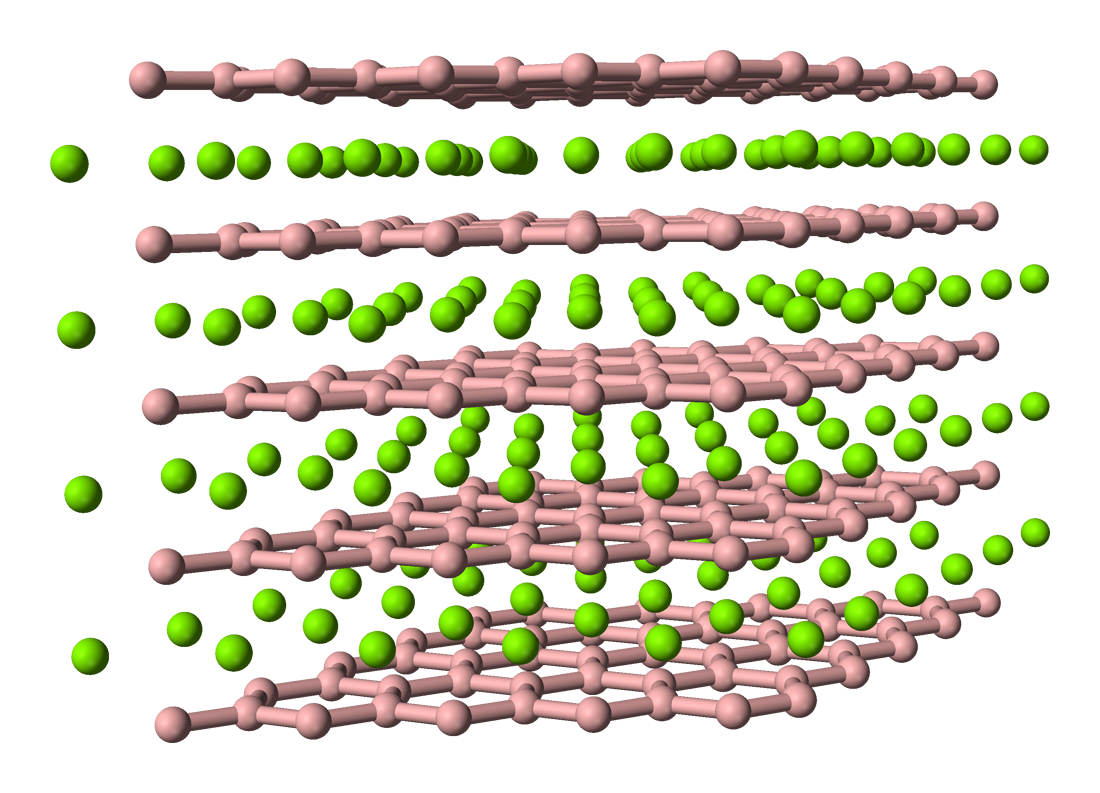
Yttrium Borides
Yttrium boride refers to a crystalline material composed of different proportions of yttrium and boron, such as YB2, YB4, YB6, YB12, YB25, YB50 and YB66. They are all gray-colored, hard solids having high melting temperatures. The most common form is the yttrium hexaboride YB6. It exhibits superconductivity at relatively high temperature of 8.4 K and, similar to LaB6, is an electron cathode. Another remarkable yttrium boride is YB66. It has a large lattice constant (2.344 nm), high thermal and mechanical stability, and therefore is used as a diffraction grating for low-energy synchrotron radiation (1–2 keV). YB2 (yttrium diboride) Yttrium diboride has the same hexagonal crystal structure as aluminium diboride and magnesium diboride – an important superconducting material. Its Pearson symbol is ''hP3'', space group P6/mmm (No 191), ''a'' = 0.33041 nm, ''c'' = 0.38465 nm and the calculated density is 5.05 g/cm3. In this structure, the boron atoms form graphite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |