|
Burimamide
Burimamide is an antagonist at the H2 and H3 histamine receptors. It is largely inactive as an H2 antagonist at physiological pH, but its H3 affinity is 100x higher. It is a thiourea derivative. Burimamide was first developed by scientists at Smith, Kline & French (SK&F; now GlaxoSmithKline) in their intent to develop a histamine antagonist for the treatment of peptic ulcers. The discovery of burimamide ultimately led to the development of cimetidine (Tagamet). See also * Metiamide * Cimetidine Cimetidine, sold under the brand name Tagamet among others, is a histamine H2 receptor antagonist that inhibits stomach acid production. It is mainly used in the treatment of heartburn and peptic ulcers. The development of longer-acting H2 rec ... References H2 receptor antagonists H3 receptor antagonists Imidazoles Thioureas {{gastrointestinal-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metiamide
Metiamide is a histamine H2 receptor antagonist developed from another H2 antagonist, burimamide. It was an intermediate compound in the development of the successful anti-ulcer drug cimetidine (Tagamet). Development of metiamide from burimamide After discovering that burimamide is largely inactive at physiological pH, due to the presence of its electron-donating side chain, the following steps were undertaken to stabilize burimamide: * addition of a sulfide group close to the imidazole ring, giving thiaburimamide * addition of methyl group to the 4-position on the imidazole ring to favor the tautomer of thiaburimamide which binds better to the H2 receptor These changes increased the bioavailability metiamide so that it is ten times more potent than burimamide in inhibiting histamine-stimulated release of gastric acid. The clinical trials that began in 1973 demonstrated the ability of metiamide to provide symptomatic relief for ulcerous patients by increasing healing rate of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
H2 Receptor Antagonists
H2 antagonists, sometimes referred to as H2RAs and also called H2 blockers, are a class of medications that block the action of histamine at the histamine H2 receptors of the parietal cells in the stomach. This decreases the production of stomach acid. H2 antagonists can be used in the treatment of dyspepsia, peptic ulcers and gastroesophageal reflux disease. They have been surpassed by proton pump inhibitors (PPIs); the PPI omeprazole was found to be more effective at both healing and alleviating symptoms of ulcers and reflux oesophagitis than the H2 blockers ranitidine and cimetidine. H2 antagonists are a type of antihistamine, although in common use the term "antihistamine" is often reserved for H1 antagonists, which relieve allergic reactions. Like the H1 antagonists, some H2 antagonists function as inverse agonists rather than receptor antagonists, due to the constitutive activity of these receptors. The prototypical H2 antagonist, called cimetidine, was developed by Si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cimetidine
Cimetidine, sold under the brand name Tagamet among others, is a histamine H2 receptor antagonist that inhibits stomach acid production. It is mainly used in the treatment of heartburn and peptic ulcers. The development of longer-acting H2 receptor antagonists with fewer drug interactions and adverse effects, such as ranitidine and famotidine, decreased the use of cimetidine, and though it is still used, cimetidine is no longer among the more widely used of the H2 receptor antagonists. Cimetidine was developed in 1971 and came into commercial use in 1977. Cimetidine was approved in the United Kingdom in 1976, and was approved in the United States by the Food and Drug Administration for prescriptions in 1979. Medical uses Cimetidine is used to inhibit stomach acid production and is used in the treatment of heartburn and peptic ulcers. Other uses Some evidence suggests cimetidine could be effective in the treatment of common warts, but more rigorous double-blind clinical t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cimetidine
Cimetidine, sold under the brand name Tagamet among others, is a histamine H2 receptor antagonist that inhibits stomach acid production. It is mainly used in the treatment of heartburn and peptic ulcers. The development of longer-acting H2 receptor antagonists with fewer drug interactions and adverse effects, such as ranitidine and famotidine, decreased the use of cimetidine, and though it is still used, cimetidine is no longer among the more widely used of the H2 receptor antagonists. Cimetidine was developed in 1971 and came into commercial use in 1977. Cimetidine was approved in the United Kingdom in 1976, and was approved in the United States by the Food and Drug Administration for prescriptions in 1979. Medical uses Cimetidine is used to inhibit stomach acid production and is used in the treatment of heartburn and peptic ulcers. Other uses Some evidence suggests cimetidine could be effective in the treatment of common warts, but more rigorous double-blind clinical t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, |
Histamine H2 Receptor
H2 receptors are positively coupled to adenylate cyclase via Gs. It is a potent stimulant of cAMP production, which leads to activation of protein kinase A. PKA functions to phosphorylate certain proteins, affecting their activity. The drug betazole is an example of a histamine H2 receptor agonist. Function Histamine is a ubiquitous messenger molecule released from mast cells, enterochromaffin-like cells, and neurons. Its various actions are mediated by histamine receptors H1, H2, H3 and H4. The histamine receptor H2 belongs to the rhodopsin-like family of G protein-coupled receptors. It is an integral membrane protein and stimulates gastric acid secretion. It also regulates gastrointestinal motility and intestinal secretion and is thought to be involved in regulating cell growth and differentiation. Histamine may play a role in penile erection. Tissue distribution Histamine H2 receptors are expressed in the following tissues: ;Peripheral tissues *Gastric parietal cells (oxyn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histamine H3 Receptor
Histamine H3 receptors are expressed in the central nervous system and to a lesser extent the peripheral nervous system, where they act as autoreceptors in presynaptic histaminergic neurons and control histamine turnover by feedback inhibition of histamine synthesis and release. The H3 receptor has also been shown to presynaptically inhibit the release of a number of other neurotransmitters (i.e. it acts as an inhibitory heteroreceptor) including, but probably not limited to dopamine, Gamma-aminobutyric acid, GABA, acetylcholine, noradrenaline, histamine and serotonin. The gene sequence for H3 receptors expresses only about 22% and 20% homology with both H1 and H2 receptors respectively. There is much interest in the histamine H3 receptor as a potential therapeutic target because of its involvement in the neuronal mechanism behind many cognitive disorders and especially its location in the central nervous system.Rapanelli, Maximiliano. “The Magnificent Two: Histamine and the H3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histamine
Histamine is an organic nitrogenous compound involved in local immune responses, as well as regulating physiological functions in the gut and acting as a neurotransmitter for the brain, spinal cord, and uterus. Since histamine was discovered in 1910, it has been considered a local hormone (autocoid) because it lacks the classic endocrine glands to secrete it; however, in recent years, histamine has been recognized as a central neurotransmitter. Histamine is involved in the inflammatory response and has a central role as a mediator of itching. As part of an immune response to foreign pathogens, histamine is produced by basophils and by mast cells found in nearby connective tissues. Histamine increases the permeability of the capillaries to white blood cells and some proteins, to allow them to engage pathogens in the infected tissues. It consists of an imidazole ring attached to an ethylamine chain; under physiological conditions, the amino group of the side-chain is protonate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor (biochemistry)
In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and cause some form of cellular/tissue response, e.g. a change in the electrical activity of a cell. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand, and integration allows the signal to be incorporated into another biochemical pathway. Receptor proteins can be classified by their location. Transmembrane receptors include ligand-gated ion channels, G protein-coupled receptors, and enzyme-linked hormone receptors. Intracellular receptors are those found inside the cell, and include cytoplasmic receptors and nuclear receptors. A molecule that binds to a receptor is called a ligand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiourea
Thiourea () is an organosulfur compound with the formula and the structure . It is structurally similar to urea (), except that the oxygen atom is replaced by a sulfur atom (as implied by the ''thio-'' prefix); however, the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refer to a broad class of compounds with the general structure . Thioureas are related to thioamides, e.g. , where R is methyl, ethyl, etc. Structure and bonding Thiourea is a planar molecule. The C=S bond distance is 1.71 Å. The C-N distances average 1.33 Å. The weakening of the C-S bond by C-N pi-bonding is indicated by the short C=S bond in thiobenzophenone, which is 1.63 Å. Thiourea occurs in two tautomeric forms, of which the thione form predominates in aqueous solutions. The equilibrium constant has been calculated as ''K''eq is . The thiol form, which is also known as an isothiourea, can be encountered in substituted compounds such as i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GlaxoSmithKline
GSK plc, formerly GlaxoSmithKline plc, is a British multinational pharmaceutical and biotechnology company with global headquarters in London, England. Established in 2000 by a merger of Glaxo Wellcome and SmithKline Beecham. GSK is the tenth largest pharmaceutical company and #294 on the 2022 ''Fortune'' Global 500, ranked behind other pharmaceutical companies China Resources, Sinopharm, Johnson & Johnson, Pfizer, Roche, AbbVie, Novartis, Bayer, and Merck. The company has a primary listing on the London Stock Exchange and is a constituent of the FTSE 100 Index. , it had a market capitalisation of £70 billion, the eighth largest on the London Stock Exchange. It has a secondary listing on the New York Stock Exchange. The company developed the first malaria vaccine, RTS,S, which it said in 2014 it would make available for five percent above cost. Legacy products developed at GSK include several listed in the World Health Organization's List of Essential Medicines, such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
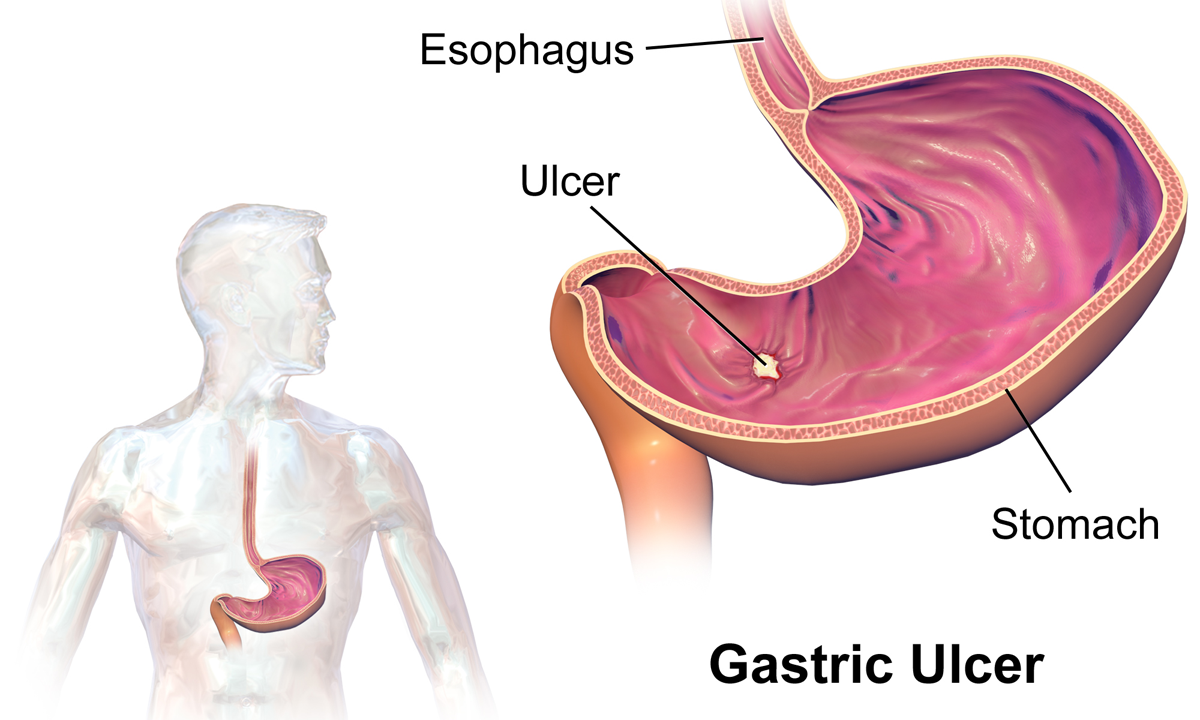
Peptic Ulcers
Peptic ulcer disease (PUD) is a break in the inner lining of the stomach, the first part of the small intestine, or sometimes the lower esophagus. An ulcer in the stomach is called a gastric ulcer, while one in the first part of the intestines is a duodenal ulcer. The most common symptoms of a duodenal ulcer are waking at night with upper abdominal pain and upper abdominal pain that improves with eating. With a gastric ulcer, the pain may worsen with eating. The pain is often described as a burning or dull ache. Other symptoms include belching, vomiting, weight loss, or poor appetite. About a third of older people have no symptoms. Complications may include bleeding, perforation, and blockage of the stomach. Bleeding occurs in as many as 15% of cases. Common causes include the bacteria ''Helicobacter pylori'' and non-steroidal anti-inflammatory drugs (NSAIDs). Other, less common causes include tobacco smoking, stress as a result of other serious health conditions, Behçet's di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |