|
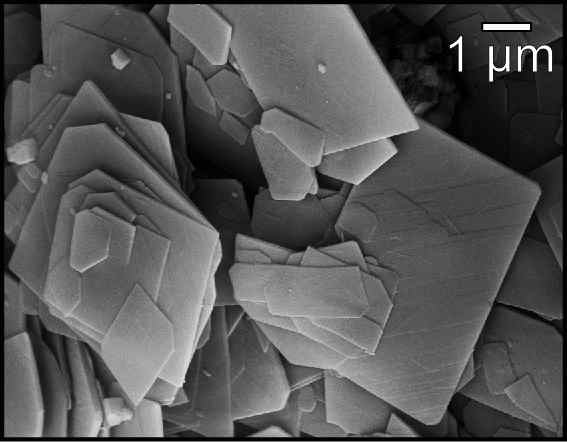
Brucite
Brucite is the mineral form of magnesium hydroxide, with the chemical formula Mg( OH)2. It is a common alteration product of periclase in marble; a low-temperature hydrothermal vein mineral in metamorphosed limestones and chlorite schists; and formed during serpentinization of dunites. Brucite is often found in association with serpentine, calcite, aragonite, dolomite, magnesite, hydromagnesite, artinite, talc and chrysotile. It adopts a layered CdI2-like structure with hydrogen-bonds between the layers. Discovery Brucite was first described in 1824 by François Sulpice Beudant and named for the discoverer, American mineralogist, Archibald Bruce (1777–1818). A fibrous variety of brucite is called nemalite. It occurs in fibers or laths, usually elongated along 010 but sometimes 120 crystalline directions. Occurrence A notable location in the U.S. is Wood's Chrome Mine, Cedar Hill Quarry, Lancaster County, Pennsylvania. Yellow, white and blue Brucite with a botryoidal habi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serpentinization
Serpentinization is a hydration and metamorphic transformation of ferromagnesian minerals, such as olivine and pyroxene, in mafic and ultramafic rock to produce serpentinite. Minerals formed by serpentinization include the serpentine group minerals (antigorite, lizardite, chrysotile), brucite, talc, Ni-Fe alloys, and magnetite. The mineral alteration is particularly important at the sea floor at tectonic plate boundaries. Formation and petrology Serpentinization is a form of low-temperature (0 to ~600 °C) metamorphism of ferromagnesian minerals in mafic and ultramafic rocks, such as dunite, harzburgite, or lherzolite. These are rocks low in silica and composed mostly of olivine (), pyroxene (), and chromite (approximately ). Serpentinization is driven largely by hydration and oxidation of olivine and pyroxene to serpentine group minerals (antigorite, lizardite, and chrysotile), brucite (), talc (Mg3Si4O10(OH)2, and magnetite (). Under the unusual chemical conditions acc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Hydroxide
Magnesium hydroxide is the inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (). Magnesium hydroxide is a common component of antacids, such as milk of magnesia. Preparation Treating the solution of different soluble magnesium salts with alkaline water induces the precipitation of the solid hydroxide Mg(OH)2: :Mg2+ + 2OH− → Mg(OH)2 As is the second most abundant cation present in seawater after , it can be economically extracted directly from seawater by alkalinisation as described here above. On an industrial scale, Mg(OH)2 is produced by treating seawater with lime (Ca(OH)2). A volume of (or 160,000 US gallons) of seawater gives about one ton of Mg(OH)2. Ca(OH)2 is far more soluble than Mg(OH)2 and drastically increases the pH value of seawater from 8.2 to 12.5. The less soluble precipitates because of the common ion effect due to the added by the dissolution of : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorite Group
The chlorites are the group of phyllosilicate minerals common in low-grade metamorphic rocks and in altered igneous rocks. Greenschist, formed by metamorphism of basalt or other low-silica volcanic rock, typically contains significant amounts of chlorite. Chlorite minerals show a wide variety of compositions, in which magnesium, iron, aluminium, and silicon substitute for each other in the crystal structure. A complete solid solution series exists between the two most common end members, magnesium-rich clinochlore and iron-rich chamosite. In addition, manganese, zinc, lithium, and calcium species are known. The great range in composition results in considerable variation in physical, optical, and X-ray properties. Similarly, the range of chemical composition allows chlorite group minerals to exist over a wide range of temperature and pressure conditions. For this reason chlorite minerals are ubiquitous minerals within low and medium temperature metamorphic rocks, some igneous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Talc
Talc, or talcum, is a clay mineral, composed of hydrated magnesium silicate with the chemical formula Mg3Si4O10(OH)2. Talc in powdered form, often combined with corn starch, is used as baby powder. This mineral is used as a thickening agent and lubricant. It is an ingredient in ceramics, paints, and roofing material. It is a main ingredient in many cosmetics. It occurs as foliated to fibrous masses, and in an exceptionally rare crystal form. It has a perfect basal cleavage and an uneven flat fracture, and it is foliated with a two-dimensional platy form. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 1 as the hardness of talc, the softest mineral. When scraped on a streak plate, talc produces a white streak; though this indicator is of little importance, because most silicate minerals produce a white streak. Talc is translucent to opaque, with colors ranging from whitish grey to green with a vitreous and pearly luster. Talc i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydromagnesite
Hydromagnesite is a hydrated magnesium carbonate mineral with the formula Mg5(CO3)4(OH)2·4H2O. It generally occurs associated with the weathering products of magnesium containing minerals such as serpentine or brucite. It occurs as incrustations and vein or fracture fillings in ultramafic rocks and serpentinites, and occurs in hydrothermally altered dolomite and marble. Hydromagnesite commonly appears in caves as speleothems and " moonmilk", deposited from water that has seeped through magnesium rich rocks. It is the most common cave carbonate after calcite and aragonite. The mineral thermally decomposes, over a temperature range of approximately 220 °C to 550 °C, releasing water and carbon dioxide leaving a magnesium oxide residue. Hydromagnesite was first described in 1836 for an occurrence in Hoboken, New Jersey. Stromatolites in an alkaline ( pH greater than 9) freshwater lake ( Salda Gölü) in southern Turkey are made of hydromagnesite precipitated by diatom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Artinite
Artinite is a hydrated magnesium carbonate mineral with formula: Mg2(CO3)(OH)2·3H2O. It forms white silky monoclinic prismatic crystals that are often in radial arrays or encrustations. It has a Mohs hardness of 2.5 and a specific gravity of 2. It occurs in low-temperature hydrothermal veins and in serpentinized ultramafic rocks. Associated minerals include brucite, hydromagnesite, pyroaurite, chrysotile, aragonite, calcite, dolomite and magnesite. It was first reported in 1902 in Lombardy, Italy Italy ( it, Italia ), officially the Italian Republic, ) or the Republic of Italy, is a country in Southern Europe. It is located in the middle of the Mediterranean Sea, and its territory largely coincides with the homonymous geographical .... It was named for Italian mineralogist, Ettore Artini (1866–1928). References Magnesium minerals Carbonate minerals Monoclinic minerals Minerals in space group 12 {{carbonate-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periclase
Periclase is a magnesium mineral that occurs naturally in contact metamorphic rocks and is a major component of most basic refractory bricks. It is a cubic form of magnesium oxide ( Mg O). In nature it usually forms a solid solution with wüstite (FeO) and is then referred to as ferropericlase or magnesiowüstite. It was first described in 1840 and named from the Greek περικλάω (to break around) in allusion to its cleavage. The type locality is Monte Somma, Somma-Vesuvius Complex, Naples Province, Campania, Italy. The old term for the mineral is ''magnesia''. Stones from the Magnesia region in ancient Anatolia contained both magnesium oxide and hydrated magnesium carbonate as well as iron oxides (such as magnetite). Thus these stones, called ''Stones from Magnesia'' in antiquity, with their unusual magnetic properties were the reason the terms ''magnet'' and ''magnetism'' were coined. Periclase is usually found in marble produced by metamorphism of dolomitic limest ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Minerals'; p. 1. In the series ''Geology: Landforms, Minerals, and Rocks''. Rosen Publishing Group. The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or are organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks. The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral, or may be an aggregate of two or more different types of minerals, spacially segregated into disti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Archibald Bruce (mineralogist)
Archibald Bruce (February 1777 – February 22, 1818) was an American physician and mineralogist. Biography Bruce was born in New York City in February 1777 and graduated from Columbia College in 1795 with a Bachelor of Arts degree. His father, William Bruce, head of the medical department of the British army at New York, on being ordered to the West Indies, specially directed that his son should not be brought up to the medical profession. From the medical lectures of Nicholas Romayne, the teachings of Dr. Hosack, and attendance on the courses of medical instruction of Columbia, he attained a knowledge of the science. He went to Europe in 1798, received an M.D. from the University of Edinburgh in 1800, and, in a tour of two years in France, Switzerland, and Italy, collected a mineralogical cabinet of great value. He married in London, and in the summer of 1803, returned to New York city and began practice. In 1807, he was appointed professor of materia medica and mineralog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German ''Calcit'', a term from the 19th century that came from the Latin word for lime, ''calx'' (genitive calcis) with the suffix "-ite" used to name minerals. It is thus etymologically related to chalk. When applied by archaeologists and stone trade professionals, the term alabaster is used not just as in geology and mineralogy, where it is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
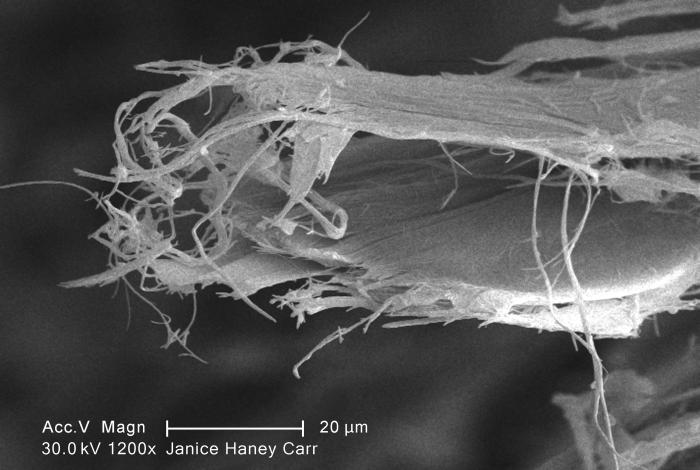
Chrysotile
Chrysotile or white asbestos is the most commonly encountered form of asbestos, accounting for approximately 95% of the asbestos in the United States Occupational Safety and Health Administration, U.S. Department of Labor (2007)29 C.F.R. 1910.1001 Appendix J. and a similar proportion in other countries.Institut national de recherche sur la sécurité (1997).Amiante." ''Fiches toxicologiques.'' n° 167. (in French) It is a soft, fibrous silicate mineral in the serpentine subgroup of phyllosilicates; as such, it is distinct from other asbestiform minerals in the amphibole group. Its idealized chemical formula is Mg( Si O)( OH). The material has physical properties which make it desirable for inclusion in building materials, but poses serious health risks when dispersed into air and inhaled. Polytypes Three polytypes of chrysotile are known. These are very difficult to distinguish in hand specimens, and polarized light microscopy must normally be used. Some older ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |